Effectiveness and medication acceptance of olanzapine disintegrating tablets compared to standard olanzapine tablets in acutely treated psychiatric patients
- PMID: 19956444
- PMCID: PMC2779125
- DOI: 10.2147/ppa.s2300
Effectiveness and medication acceptance of olanzapine disintegrating tablets compared to standard olanzapine tablets in acutely treated psychiatric patients
Abstract
Background: This study compared effectiveness and acceptance of orally disintegrating olanzapine tablets (ODT) with standard-coated tablets (SOT) in acutely ill psychiatric patients admitted to psychiatric hospitals for emergency treatment.
Methods: Large, prospective, observational study at hospital emergency units across Germany in patients with a diagnosis or tentative diagnosis of acute schizophrenia treated with ODT or SOT. Clinical (CGI-S and CGI-I) outcomes, attitudes towards medication (Nursing Assessment of Medication Acceptance, NAMA) scale, suicidal ideation, and adverse events were assessed at start of treatment and after 2 weeks.
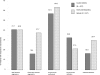
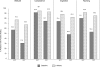
Results: Both olanzapine formulations, ODT (N = 247) and SOT (N = 207), showed similar effectiveness after 2 weeks. CGI-I improved in 92.1% of patients (ODT: 91.8%, SOT: 92.3%). In patients receiving both formulations suicidal ideations were reduced (ODT from 53.9% to 20.6%, SOT from 51.2% to 22.7%). ODT was preferably given to severely ill (SOT: 49.8%, ODT: 64.4%) and aggressive patients. Adverse events were reported for 6.5% of ODT- and 2.9% of SOT-patients. This difference was possibly caused by the characteristics of patients receiving ODT.
Conclusions: This non-randomized, observational study shows comparable outcomes and tolerability in patients treated with both olanzapine formulations. In an acute treatment setting, orally disintegrating tablets were preferably used for more severely ill and aggressive patients with low medication acceptance.
Keywords: acute treatment; observational study; olanzapine; schizophrenia.
Figures

References
-
- Alvarez E, Bobes J, Gomez J-C, et al. Safety of olanzapine versus conventional antipsychotics in the treatment of patients with acute schizophrenia. A naturalistic study. Eur Neuropsychopharmacol. 2003;13:39–48. - PubMed
-
- Baker RW, Kinon BJ, Maguire GA, et al. Effectiveness of rapid initial dose escalation of up to forty milligrams per day of oral olanzapine in acute agitation. J Clin Psychopharmacol. 2003;23:342–8. - PubMed
-
- Beasley CM, Sayler ME, Kiesler GM. The influence of pharmaco-therapy on self-directed and externally-directed aggression in schizophrenia. Schizophr Res. 1998;29:28.
-
- Behnke K, Sogaard J, Martin S, et al. Mirtazapine orally disintegrating tablet versus sertaline: a prospective onset of action study. J Clin Psychopharmacol. 2003;23:358–64. - PubMed
-
- Bergstrom RF, Mitchell M, Witcher J, et al. Rapid onset of absorption with olanzapine orally disintegrating tablets. Schizophr Res. 2004;67:160.
LinkOut - more resources
Full Text Sources

