Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity
- PMID: 19956493
- PMCID: PMC2785403
- DOI: 10.4143/crt.2005.37.2.133
Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity
Abstract
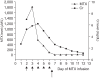
A 13 year-old girl with osteosarcoma and pulmonary tumor recurrence developed acute renal failure following high dose methotrexate (12 g/m(2)) therapy, she had previously tolerated high dose methotrexate and her renal and hepatic functions were normal. Briefly, 48 hours after beginning methotrexate infusion her methotrexate concentration and creatinine level were 1338.8 microM/L and 5.8 mg/dl, respectively. Grade IV oral mucositis and neutropenia with fever developed at 144 hours after MTX infusion. Hydration and alkalinization were continued and leucovorin rescue was intensified based on the plasma MTX concentrations. Plasma exchange was performed twice and hemodialysis 3 times without problems, but methotrexate and creatinine levels remained high, 91.9 microM/L, and 2.5 mg/dl, respectively. After 3 courses of hemodialysis carboxypeptidase-G2 (CPDG2) was administered at 50 U/kg, intravenously over 5 minutes. After 15 minutes of CPDG2 (Voraxaze) infusion, her plasma MTX concentration was 0.91 microM/L and no rebound elevation or side effects developed. Thirteen days post-MTX infusion her renal function had normalized. We report here our experience of a dramatic methotrexate level reduction caused by CPDG2 administration.
Keywords: Acute renal failure; Carboxypeptidase-G2; High dose methotrexate; Leucovorin.
Figures
References
-
- Lankelma J, van der Klein E, Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980;9:133–142. - PubMed
-
- Bleyer WA. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4:87–101. - PubMed
-
- Flombaum CD, Meyers PA. High-dose leucovorin as sole therapy for methotrexate toxicity. J Clin Oncol. 1999;17:1589–1594. - PubMed
-
- Relling MV, Stapleton FB, Ochs J, Jones DP, Meyer W, Wainer IW, et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer. 1988;62:884–888. - PubMed
-
- Kawabata K, Makino H, Nagake Y, Tokioka H, Matsumi M, Morita Y, et al. A case of methotrexate-induced acute renal failure successfully treated with plasma perfusion and sequential hemodialysis. Nephron. 1995;71:233–234. - PubMed
LinkOut - more resources
Full Text Sources