Forward models and state estimation in compensatory eye movements
- PMID: 19956563
- PMCID: PMC2786296
- DOI: 10.3389/neuro.03.013.2009
Forward models and state estimation in compensatory eye movements
Abstract
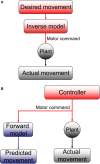
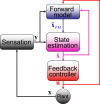
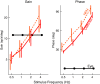
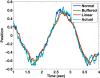
The compensatory eye movement (CEM) system maintains a stable retinal image, integrating information from different sensory modalities to compensate for head movements. Inspired by recent models of the physiology of limb movements, we suggest that CEM can be modeled as a control system with three essential building blocks: a forward model that predicts the effects of motor commands; a state estimator that integrates sensory feedback into this prediction; and, a feedback controller that translates a state estimate into motor commands. We propose a specific mapping of nuclei within the CEM system onto these control functions. Specifically, we suggest that the Flocculus is responsible for generating the forward model prediction and that the Vestibular Nuclei integrate sensory feedback to generate an estimate of current state. Finally, the brainstem motor nuclei - in the case of horizontal compensation this means the Abducens Nucleus and the Nucleus Prepositus Hypoglossi - implement a feedback controller, translating state into motor commands. While these efforts to understand the physiological control system as a feedback control system are in their infancy, there is the intriguing possibility that CEM and targeted voluntary movements use the same cerebellar circuitry in fundamentally different ways.
Keywords: cerebellum; control systems; eye movements; forward model; model; okr; vestibular nucleus; vor.
Figures




References
LinkOut - more resources
Full Text Sources

