Rearrangements of the actin cytoskeleton and E-cadherin-based adherens junctions caused by neoplasic transformation change cell-cell interactions
- PMID: 19956566
- PMCID: PMC2779654
- DOI: 10.1371/journal.pone.0008027
Rearrangements of the actin cytoskeleton and E-cadherin-based adherens junctions caused by neoplasic transformation change cell-cell interactions
Abstract
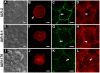
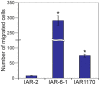
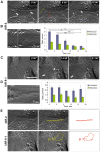
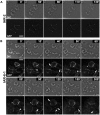
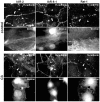
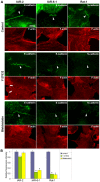
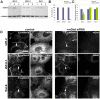
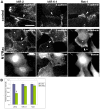
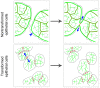
E-cadherin-mediated cell-cell adhesion, which is essential for the maintenance of the architecture and integrity of epithelial tissues, is often lost during carcinoma progression. To better understand the nature of alterations of cell-cell interactions at the early stages of neoplastic evolution of epithelial cells, we examined the line of nontransformed IAR-2 epithelial cells and their descendants, lines of IAR-6-1 epithelial cells transformed with dimethylnitrosamine and IAR1170 cells transformed with N-RasG12D. IAR-6-1 and IAR1170 cells retained E-cadherin, displayed discoid or polygonal morphology, and formed monolayers similar to IAR-2 monolayer. Fluorescence staining, however, showed that in IAR1170 and IAR-6-1 cells the marginal actin bundle, which is typical of nontransformed IAR-2 cells, disappeared, and the continuous adhesion belt (tangential adherens junctions (AJs)) was replaced by radially oriented E-cadherin-based AJs. Time-lapse imaging of IAR-6-1 cells stably transfected with GFP-E-cadherin revealed that AJs in transformed cells are very dynamic and unstable. The regulation of AJ assembly by Rho family small GTPases was different in nontransformed and in transformed IAR epithelial cells. As our experiments with the ROCK inhibitor Y-27632 and the myosin II inhibitor blebbistatin have shown, the formation and maintenance of radial AJs critically depend on myosin II-mediated contractility. Using the RNAi technique for the depletion of mDia1 and loading cells with N17Rac, we established that mDia1 and Rac are involved in the assembly of tangential AJs in nontransformed epithelial cells but not in radial AJs in transformed cells. Neoplastic transformation changed cell-cell interactions, preventing contact paralysis after the establishment of cell-cell contact and promoting dynamic cell-cell adhesion and motile behavior of cells. It is suggested that the disappearance of the marginal actin bundle and rearrangements of AJs may change the adhesive function of E-cadherin and play an active role in migratory activity of carcinoma cells.
Conflict of interest statement
Figures


References
-
- Abercrombie M. Contact inhibition in tissue culture. In Vitro. 1970;6:128–142. - PubMed
-
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

