Gender differences in a Drosophila transcriptomic model of chronic pentylenetetrazole induced behavioral deficit
- PMID: 19956579
- PMCID: PMC2779862
- DOI: 10.1371/journal.pone.0008136
Gender differences in a Drosophila transcriptomic model of chronic pentylenetetrazole induced behavioral deficit
Abstract
A male Drosophila model of locomotor deficit induced by chronic pentylenetetrazole (PTZ), a proconvulsant used to model epileptogenesis in rodents, has recently been described. Antiepileptic drugs (AEDs) ameliorate development of this behavioral abnormality. Time-series of microarray profiling of heads of male flies treated with PTZ has shown epileptogenesis-like transcriptomic perturbation in the fly model. Gender differences are known to exist in neurological and psychiatric conditions including epileptogenesis. We describe here the effects of chronic PTZ in Drosophila females, and compare the results with the male model. As in males, chronic PTZ was found to cause a decreased climbing speed in females. In males, overrepresentation of Wnt, MAPK, TGF-beta, JAK-STAT, Cell communication, and Dorso-Ventral axis formation pathways in downregulated genes was previously described. Of these, female genes showed enrichment only for Dorso-Ventral axis formation. Surprisingly, the ribosomal pathway was uniquely overrepresented in genes downregulated in females. Gender differences thus exist in the Drosophila model. Gender neutral, the developmental pathway Dorso-Ventral axis formation may be considered as the candidate causal pathway in chronic pentylenetetrazole induced behavioral deficit. Prior evidence of developmental mechanisms in epileptogenesis may support potential usefulness of the fly model. Given this, gender specific pathways identified here may provide a lead for further understanding brain dimorphism in neuropsychiatric disorders.
Conflict of interest statement
Figures
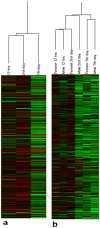

References
-
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–960. - PubMed
-
- Nguon K, Ladd B, Baxter MG, Sajdel-Sulkowska EM. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res. 2005;148:341–351. - PubMed
-
- Nickel J, Jokeit H, Wunderlich G, Ebner A, Witte OW, et al. Gender-specific differences of hypometabolism in mTLE: implication for cognitive impairments. Epilepsia. 2003;44:1551–1561. - PubMed
-
- Lemmens EM, Lubbers T, Schijns OE, Beuls EA, Hoogland G. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005;46:1603–1612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

