Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration
- PMID: 19956583
- PMCID: PMC2780296
- DOI: 10.1371/journal.pone.0008122
Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration
Abstract
Background: The adult subventricular zone (SVZ) contains stem and progenitor cells that generate neuroblasts throughout life. Although it is well accepted that SVZ neuroblasts are migratory, recent evidence suggests their progenitor cells may also exhibit motility. Since stem and progenitor cells are proliferative and multipotential, if they were also able to move would have important implications for SVZ neurogenesis and its potential for repair.
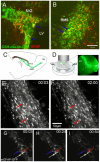
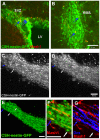
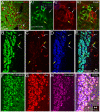
Methodology/principal findings: We studied whether SVZ stem and/or progenitor cells are motile in transgenic GFP+ slices with two photon time lapse microscopy and post hoc immunohistochemistry. We found that stem and progenitor cells; mGFAP-GFP+ cells, bright nestin-GFP+ cells and Mash1+ cells were stationary in the SVZ and rostral migratory stream (RMS). In our search for motile progenitor cells, we uncovered a population of motile betaIII-tubulin+ neuroblasts that expressed low levels of epidermal growth factor receptor (EGFr). This was intriguing since EGFr drives proliferation in the SVZ and affects migration in other systems. Thus we examined the potential role of EGFr in modulating SVZ migration. Interestingly, EGFr(low) neuroblasts moved slower and in more tortuous patterns than EGFr-negative neuroblasts. We next questioned whether EGFr stimulation affects SVZ cell migration by imaging Gad65-GFP+ neuroblasts in the presence of transforming growth factor alpha (TGF-alpha), an EGFr-selective agonist. Indeed, acute exposure to TGF-alpha decreased the percentage of motile cells by approximately 40%.
Conclusions/significance: In summary, the present study directly shows that SVZ stem and progenitor cells are static, that EGFr is retained on some neuroblasts, and that EGFr stimulation negatively regulates migration. This result suggests an additional role for EGFr signaling in the SVZ.
Conflict of interest statement
Figures



References
-
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. - PubMed
-
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

