Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis
- PMID: 19956584
- PMCID: PMC2780298
- DOI: 10.1371/journal.pone.0008130
Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a progressive and fatal motor neuron disease, and protein aggregation has been proposed as a possible pathogenetic mechanism. However, the aggregate protein constituents are poorly characterized so knowledge on the role of aggregation in pathogenesis is limited.
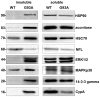
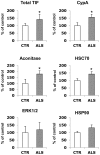
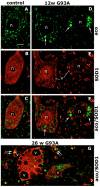
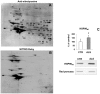
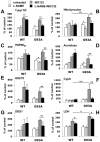
Methodology/principal findings: We carried out a proteomic analysis of the protein composition of the insoluble fraction, as a model of protein aggregates, from familial ALS (fALS) mouse model at different disease stages. We identified several proteins enriched in the detergent-insoluble fraction already at a preclinical stage, including intermediate filaments, chaperones and mitochondrial proteins. Aconitase, HSC70 and cyclophilin A were also significantly enriched in the insoluble fraction of spinal cords of ALS patients. Moreover, we found that the majority of proteins in mice and HSP90 in patients were tyrosine-nitrated. We therefore investigated the role of nitrative stress in aggregate formation in fALS-like murine motor neuron-neuroblastoma (NSC-34) cell lines. By inhibiting nitric oxide synthesis the amount of insoluble proteins, particularly aconitase, HSC70, cyclophilin A and SOD1 can be substantially reduced.
Conclusion/significance: Analysis of the insoluble fractions from cellular/mouse models and human tissues revealed novel aggregation-prone proteins and suggests that nitrative stress contribute to protein aggregate formation in ALS.
Conflict of interest statement
Figures

References
-
- Ross CA, Poirier MA. What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol. 2005;6:891–898. - PubMed
-
- Strong MJ, Kesavapany S, Pant HC. The pathobiology of amyotrophic lateral sclerosis: a proteinopathy? J Neuropathol Exp Neurol. 2005;64:649–664. - PubMed
-
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. - PubMed
-
- Durham HD, Roy J, Dong L, Figlewicz DA. Aggregation of mutant Cu/Zn superoxide dismutase proteins in a culture model of ALS. J Neuropathol Exp Neurol. 1997;56:523–530. - PubMed
-
- Basso M, Massignan T, Samengo G, Cheroni C, De Biasi S, et al. Insoluble mutant SOD1 is partly oligoubiquitinated in amyotrophic lateral sclerosis mice. J Biol Chem. 2006;281:33325–33335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

