Fetuin-A/albumin-mineral complexes resembling serum calcium granules and putative nanobacteria: demonstration of a dual inhibition-seeding concept
- PMID: 19956594
- PMCID: PMC2779105
- DOI: 10.1371/journal.pone.0008058
Fetuin-A/albumin-mineral complexes resembling serum calcium granules and putative nanobacteria: demonstration of a dual inhibition-seeding concept
Abstract
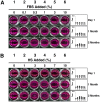
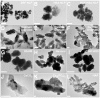
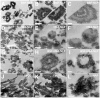
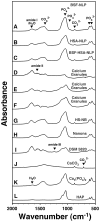
Serum-derived granulations and purported nanobacteria (NB) are pleomorphic apatite structures shown to resemble calcium granules widely distributed in nature. They appear to be assembled through a dual inhibitory-seeding mechanism involving proteinaceous factors, as determined by protease (trypsin and chymotrypsin) and heat inactivation studies. When inoculated into cell culture medium, the purified proteins fetuin-A and albumin fail to induce mineralization, but they will readily combine with exogenously added calcium and phosphate, even in submillimolar amounts, to form complexes that will undergo morphological transitions from nanoparticles to spindles, films, and aggregates. As a mineralization inhibitor, fetuin-A is much more potent than albumin, and it will only seed particles at higher mineral-to-protein concentrations. Both proteins display a bell-shaped, dose-dependent relationship, indicative of the same dual inhibitory-seeding mechanism seen with whole serum. As ascertained by both seeding experiments and gel electrophoresis, fetuin-A is not only more dominant but it appears to compete avidly for nanoparticle binding at the expense of albumin. The nanoparticles formed in the presence of fetuin-A are smaller than their albumin counterparts, and they have a greater tendency to display a multi-layered ring morphology. In comparison, the particles seeded by albumin appear mostly incomplete, with single walls. Chemically, spectroscopically, and morphologically, the protein-mineral particles resemble closely serum granules and NB. These particles are thus seen to undergo an amorphous to crystalline transformation, the kinetics and completeness of which depend on the protein-to-mineral ratios, with low ratios favoring faster conversion to crystals. Our results point to a dual inhibitory-seeding, de-repression model for the assembly of particles in supersaturated solutions like serum. The presence of proteins and other inhibitory factors tend to block apatite nuclei formation or to stabilize the nascent nuclei as amorphous or semi-crystalline spherical nanoparticles, until the same inhibitory influences are overwhelmed or de-repressed, whereby the apatite nuclei grow in size to coalesce into crystalline spindles and films-a mechanism that may explain not only the formation of calcium granules in nature but also normal or ectopic calcification in the body.
Conflict of interest statement
Figures










References
-
- Kajander EO, Kuronen I, Akerman K, Pelttari A, Ciftcioglu N. Nanobacteria from blood, the smallest culturable autonomously replicating agent on Earth. Proc Soc Photo Opt Instrum Eng. 1997;3111:420–428.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

