Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis
- PMID: 19956603
- PMCID: PMC2779453
- DOI: 10.1371/journal.pone.0008013
Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis
Abstract
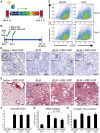
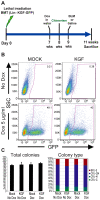
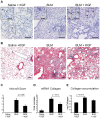
Many common diseases of the gas exchange surface of the lung have no specific treatment but cause serious morbidity and mortality. Idiopathic Pulmonary Fibrosis (IPF) is characterized by alveolar epithelial cell injury, interstitial inflammation, fibroblast proliferation and collagen accumulation within the lung parenchyma. Keratinocyte Growth Factor (KGF, also known as FGF-7) is a critical mediator of pulmonary epithelial repair through stimulation of epithelial cell proliferation. During repair, the lung not only uses resident cells after injury but also recruits circulating bone marrow-derived cells (BMDC). Several groups have used Mesenchymal Stromal Cells (MSCs) as therapeutic vectors, but little is known about the potential of Hematopoietic Stem cells (HSCs). Using an inducible lentiviral vector (Tet-On) expressing KGF, we were able to efficiently transduce both MSCs and HSCs, and demonstrated that KGF expression is induced in a regulated manner both in vitro and in vivo. We used the in vivo bleomycin-induced lung fibrosis model to assess the potential therapeutic effect of MSCs and HSCs. While both populations reduced the collagen accumulation associated with bleomycin-induced lung fibrosis, only transplantation of transduced HSCs greatly attenuated the histological damage. Using double immunohistochemistry, we show that the reduced lung damage likely occurs through endogenous type II pneumocyte proliferation induced by KGF. Taken together, our data indicates that bone marrow transplantation of lentivirus-transduced HSCs can attenuate lung damage, and shows for the first time the potential of using an inducible Tet-On system for cell based gene therapy in the lung.
Conflict of interest statement
Figures


References
-
- Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest. 1984;50:487–488. - PubMed
-
- Harrison JH, Jr., Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther. 1987;243:1185–1194. - PubMed
-
- Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

