Humoral immune response to mixed PfAMA1 alleles; multivalent PfAMA1 vaccines induce broad specificity
- PMID: 19956619
- PMCID: PMC2779588
- DOI: 10.1371/journal.pone.0008110
Humoral immune response to mixed PfAMA1 alleles; multivalent PfAMA1 vaccines induce broad specificity
Abstract
Apical Membrane Antigen 1 (AMA1), a merozoite protein essential for red cell invasion, is a candidate malaria vaccine component. Immune responses to AMA1 can protect in experimental animal models and antibodies isolated from AMA1-vaccinated or malaria-exposed humans can inhibit parasite multiplication in vitro. The parasite is haploid in the vertebrate host and the genome contains a single copy of AMA1, yet on a population basis a number of AMA1 molecular surface residues are polymorphic, a property thought to be primarily as a result of selective immune pressure. After immunisation with AMA1, antibodies more effectively inhibit strains carrying homologous AMA1 genes, suggesting that polymorphism may compromise vaccine efficacy. Here, we analyse induction of broad strain inhibitory antibodies with a multi-allele Plasmodium falciparum AMA1 (PfAMA1) vaccine, and determine the relative importance of cross-reactive and strain-specific IgG fractions by competition ELISA and in vitro parasite growth inhibition assays. Immunisation of rabbits with a PfAMA1 allele mixture yielded an increased proportion of antibodies to epitopes common to all vaccine alleles, compared to single allele immunisation. Competition ELISA with the anti-PfAMA1 antibody fraction that is cross-reactive between FVO and 3D7 AMA1 alleles showed that over 80% of these common antibodies were shared with other PfAMA1 alleles. Furthermore, growth inhibition assays revealed that for any PfAMA1 allele (FVO or 3D7), the cross-reactive fraction alone, on basis of weight, had the same functional capacity on homologous parasites as the total affinity-purified IgGs (cross-reactive+strain-specific). By contrast, the strain-specific IgG fraction of either PfAMA1 allele showed slightly less inhibition of red cell invasion by homologous strains. Thus multi-allele immunisation relatively increases the levels of antibodies to common allele epitopes. This explains the broadened cross inhibition of diverse malaria parasites, and suggests multi-allele approaches warrant further clinical investigation.
Conflict of interest statement
Figures
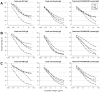
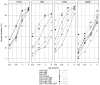

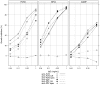
References
-
- WHO pp. 1–215. (2008) World malaria Report 2008.
-
- Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008;24:74–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

