Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops
- PMID: 19956622
- PMCID: PMC2779592
- DOI: 10.1371/journal.pone.0008108
Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops
Abstract
Background: The development of a gonorrhea vaccine is challenged by the lack of correlates of protection. The antigenically variable neisserial opacity (Opa) proteins are expressed during infection and have a semivariable (SV) and highly conserved (4L) loop that could be targeted in a vaccine. Here we compared antibodies to linear (Ab(linear)) and cyclic (Ab(cyclic)) peptides that correspond to the SV and 4L loops and selected hypervariable (HV(2)) loops for surface-binding and protective activity in vitro and in vivo.
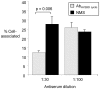
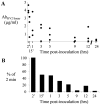
Methods/findings: Ab(SV cyclic) bound a greater number of different Opa variants than Ab(SV linear), including variants that differed by seven amino acids. Antibodies to the 4L peptide did not bind Opa-expressing bacteria. Ab(SV) (cyclic) and Ab(HV2) (cyclic), but not Ab(SV) (linear) or Ab(HV2 linear) agglutinated homologous Opa variants, and Ab(HV2BD) (cyclic) but not Ab(HV2BD) (linear) blocked the association of OpaB variants with human endocervical cells. Only Ab(HV2BD) (linear) were bactericidal against the serum resistant parent strain. Consistent with host restrictions in the complement cascade, the bactericidal activity of Ab(HV2BD) (linear) was increased 8-fold when rabbit complement was used. None of the antibodies was protective when administered vaginally to mice. Antibody duration in the vagina was short-lived, however, with <50% of the antibodies recovered 3 hrs post-administration.
Conclusions: We conclude that an SV loop-specific cyclic peptide can be used to induce antibodies that recognize a broad spectrum of antigenically distinct Opa variants and have agglutination abilities. HV(2) loop-specific cyclic peptides elicited antibodies with agglutination and adherence blocking abilities. The use of human complement when testing the bactericidal activity of vaccine-induced antibodies against serum resistant gonococci is also important.
Conflict of interest statement
Figures





References
-
- McNabb SJ, Jajosky RA, Hall-Baker PA, Adams DA, Sharp P, et al. Summary of notifiable diseases–United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;55:1–92. - PubMed
-
- Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74(Suppl 1):S12–16. - PubMed
-
- Hook EW, Handsfield HH. Gonococcal Infections in the adult. In: Holmes KK, Mardh P-A, Sparling PF, Lemon SM, Stamm WE, et al., editors. Sexually Transmitted Diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 451–466.
-
- Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. - PubMed
-
- Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health. 2004;36:11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

