Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of Huntington's disease
- PMID: 19956633
- PMCID: PMC2778556
- DOI: 10.1371/journal.pone.0008025
Formation of polyglutamine inclusions in a wide range of non-CNS tissues in the HdhQ150 knock-in mouse model of Huntington's disease
Abstract
Background: Huntington's disease (HD) is an inherited progressive neurodegenerative disorder caused by a CAG repeat expansion in the ubiquitously expressed HD gene resulting in an abnormally long polyglutamine repeat in the huntingtin protein. Polyglutamine inclusions are a hallmark of the neuropathology of HD. We have previously shown that inclusion pathology is also present in the peripheral tissues of the R6/2 mouse model of HD which expresses a small N-terminal fragment of mutant huntingtin. To determine whether this peripheral pathology is a consequence of the aberrant expression of this N-terminal fragment, we extend this analysis to the genetically precise knock-in mouse model of HD, HdhQ150, which expresses mutant mouse huntingtin.
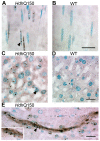
Methodology/principal findings: We have previously standardized the CAG repeat size and strain background of the R6/2 and HdhQ150 knock-in mouse models and found that they develop a comparable and widespread neuropathology. To determine whether HdhQ150 knock-in mice also develop peripheral inclusion pathology, homozygous Hdh(Q150/Q150) mice were perfusion fixed at 22 months of age, and tissues were processed for histology and immunohistochemistry with the anti-huntingtin antibody S830. The peripheral inclusion pathology was almost identical to that found in R6/2 mice at 12 weeks of age with minor differences in inclusion abundance.
Conclusions/significance: The highly comparable peripheral inclusion pathology that is present in both the R6/2 and HdhQ150 knock-in models of HD indicates that the presence of peripheral inclusions in R6/2 mice is not a consequence of the aberrant expression of an N-terminal huntingtin protein. It remains to be determined whether peripheral inclusions are a pathological feature of the human disease. Both mouse models carry CAG repeats that cause childhood disease in humans, and therefore, inclusion pathology may be a feature of the childhood rather than the adult forms of HD. It is important to establish the extent to which peripheral pathology causes the peripheral symptoms of HD from the perspective of a mechanistic understanding and future treatment options.
Conflict of interest statement
Figures




References
-
- Bates GP, Harper PS, Jones AL, editors. Oxford: Oxford University Press; 2002. Huntington's Disease. 3rd ed.
-
- Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. - PubMed
-
- Rubinsztein DC, Leggo J, Coles R, Almqvist E, Biancalana V, et al. Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats. Am J Hum Genet. 1996;59:16–22. - PMC - PubMed
-
- Henley SM, Wild EJ, Hobbs NZ, Frost C, MacManus DG, et al. Whole-brain atrophy as a measure of progression in premanifest and early Huntington's disease. Mov Disord. 2009;24:932–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

