Phosphatase-dependent regulation of epithelial mitogen-activated protein kinase responses to toxin-induced membrane pores
- PMID: 19956644
- PMCID: PMC2778951
- DOI: 10.1371/journal.pone.0008076
Phosphatase-dependent regulation of epithelial mitogen-activated protein kinase responses to toxin-induced membrane pores
Abstract
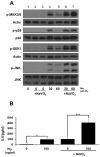
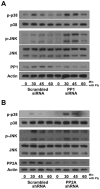
Diverse bacterial species produce pore-forming toxins (PFT) that can puncture eukaryotic cell membranes. Host cells respond to sublytic concentrations of PFT through conserved intracellular signaling pathways, including activation of mitogen-activated protein kinases (MAPK), which are critical to cell survival. Here we demonstrate that in respiratory epithelial cells p38 and JNK MAPK were phosphorylated within 30 min of exposure to pneumolysin, the PFT from Streptococcus pneumoniae. This activation was tightly regulated, and dephosphorylation of both MAPK occurred within 60 min following exposure. Pretreatment of epithelial cells with inhibitors of cellular phosphatases, including sodium orthovanadate, calyculin A, and okadaic acid, prolonged and intensified MAPK activation. Specific inhibition of MAPK phosphatase-1 did not affect the kinetics of MAPK activation in PFT-exposed epithelial cells, but siRNA-mediated knockdown of serine/threonine phosphatases PP1 and PP2A were potent inhibitors of MAPK dephosphorylation. These results indicate an important role for PP1 and PP2A in termination of epithelial responses to PFT and only a minor contribution of dual-specificity phosphatases, such as MAPK phosphatase-1, which are the major regulators of MAPK signals in other cell types. Epithelial regulation of MAPK signaling in response to membrane disruption involves distinct pathways and may require different strategies for therapeutic interventions.
Conflict of interest statement
Figures



References
-
- Iacovache I, van der Goot FG, Pernot L. Pore formation: an ancient yet complex form of attack. Biochim Biophys Acta. 2008;1778:1611–1623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

