Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes
- PMID: 19956661
- PMCID: PMC2777334
- DOI: 10.1371/journal.ppat.1000677
Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes
Abstract
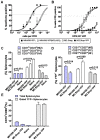
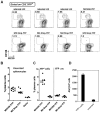
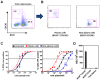
Gammaherpesviruses chronically infect their host and are tightly associated with the development of lymphoproliferative diseases and lymphomas, as well as several other types of cancer. Mechanisms involved in maintaining chronic gammaherpesvirus infections are poorly understood and, in particular, little is known about the mechanisms involved in controlling gammaherpesvirus reactivation from latently infected B cells in vivo. Recent evidence has linked plasma cell differentiation with reactivation of the human gammaherpesviruses EBV and KSHV through induction of the immediate-early viral transcriptional activators by the plasma cell-specific transcription factor XBP-1s. We now extend those findings to document a role for a gammaherpesvirus gene product in regulating plasma cell differentiation and thus virus reactivation. We have previously shown that the murine gammaherpesvirus 68 (MHV68) gene product M2 is dispensable for virus replication in permissive cells, but plays a critical role in virus reactivation from latently infected B cells. Here we show that in mice infected with wild type MHV68, virus infected plasma cells (ca. 8% of virus infected splenocytes at the peak of viral latency) account for the majority of reactivation observed upon explant of splenocytes. In contrast, there is an absence of virus infected plasma cells at the peak of latency in mice infected with a M2 null MHV68. Furthermore, we show that the M2 protein can drive plasma cell differentiation in a B lymphoma cell line in the absence of any other MHV68 gene products. Thus, the role of M2 in MHV68 reactivation can be attributed to its ability to manipulate plasma cell differentiation, providing a novel viral strategy to regulate gammaherpesvirus reactivation from latently infected B cells. We postulate that M2 represents a new class of herpesvirus gene products (reactivation conditioners) that do not directly participate in virus replication, but rather facilitate virus reactivation by manipulating the cellular milieu to provide a reactivation competent environment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Murine gammaherpesvirus 68 reactivation from B cells requires IRF4 but not XBP-1.J Virol. 2014 Oct;88(19):11600-10. doi: 10.1128/JVI.01876-14. Epub 2014 Jul 30. J Virol. 2014. PMID: 25078688 Free PMC article.
-
Lytic Replication and Reactivation from B Cells Is Not Required for Establishing or Maintaining Gammaherpesvirus Latency In Vivo.J Virol. 2022 Jun 22;96(12):e0069022. doi: 10.1128/jvi.00690-22. Epub 2022 Jun 1. J Virol. 2022. PMID: 35647668 Free PMC article.
-
Deletion of Murine Gammaherpesvirus Gene M2 in Activation-Induced Cytidine Deaminase-Expressing B Cells Impairs Host Colonization and Viral Reactivation.J Virol. 2020 Dec 9;95(1):e01933-20. doi: 10.1128/JVI.01933-20. Print 2020 Dec 9. J Virol. 2020. PMID: 33028711 Free PMC article.
-
Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner.J Virol. 2007 Jun;81(11):6134-40. doi: 10.1128/JVI.00108-07. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360749 Free PMC article. Review.
-
Murine Gammaherpesvirus 68: A Small Animal Model for Gammaherpesvirus-Associated Diseases.Adv Exp Med Biol. 2017;1018:225-236. doi: 10.1007/978-981-10-5765-6_14. Adv Exp Med Biol. 2017. PMID: 29052141 Review.
Cited by
-
Contribution of the Epstein-Barr virus to the oncogenesis of mature T-cell lymphoproliferative neoplasms.Front Oncol. 2023 Sep 14;13:1240359. doi: 10.3389/fonc.2023.1240359. eCollection 2023. Front Oncol. 2023. PMID: 37781191 Free PMC article. Review.
-
The murine gammaherpesvirus immediate-early Rta synergizes with IRF4, targeting expression of the viral M1 superantigen to plasma cells.PLoS Pathog. 2014 Aug 7;10(8):e1004302. doi: 10.1371/journal.ppat.1004302. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25101696 Free PMC article.
-
A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality.PLoS Pathog. 2011 Nov;7(11):e1002371. doi: 10.1371/journal.ppat.1002371. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22114555 Free PMC article.
-
Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells.J Virol. 2013 Jul;87(14):8004-16. doi: 10.1128/JVI.00506-13. Epub 2013 May 15. J Virol. 2013. PMID: 23678173 Free PMC article.
-
Zinc finger antiviral protein inhibits murine gammaherpesvirus 68 M2 expression and regulates viral latency in cultured cells.J Virol. 2012 Nov;86(22):12431-4. doi: 10.1128/JVI.01514-12. Epub 2012 Sep 5. J Virol. 2012. PMID: 22951821 Free PMC article.
References
-
- Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. - PubMed
-
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. - PubMed
-
- Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. - PubMed
-
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

