Identification of zoonotic genotypes of Giardia duodenalis
- PMID: 19956662
- PMCID: PMC2777335
- DOI: 10.1371/journal.pntd.0000558
Identification of zoonotic genotypes of Giardia duodenalis
Abstract
Giardia duodenalis, originally regarded as a commensal organism, is the etiologic agent of giardiasis, a gastrointestinal disease of humans and animals. Giardiasis causes major public and veterinary health concerns worldwide. Transmission is either direct, through the faecal-oral route, or indirect, through ingestion of contaminated water or food. Genetic characterization of G. duodenalis isolates has revealed the existence of seven groups (assemblages A to G) which differ in their host distribution. Assemblages A and B are found in humans and in many other mammals, but the role of animals in the epidemiology of human infection is still unclear, despite the fact that the zoonotic potential of Giardia was recognised by the WHO some 30 years ago. Here, we performed an extensive genetic characterization of 978 human and 1440 animal isolates, which together comprise 3886 sequences from 4 genetic loci. The data were assembled into a molecular epidemiological database developed by a European network of public and veterinary health Institutions. Genotyping was performed at different levels of resolution (single and multiple loci on the same dataset). The zoonotic potential of both assemblages A and B is evident when studied at the level of assemblages, sub-assemblages, and even at each single locus. However, when genotypes are defined using a multi-locus sequence typing scheme, only 2 multi-locus genotypes (MLG) of assemblage A and none of assemblage B appear to have a zoonotic potential. Surprisingly, mixtures of genotypes in individual isolates were repeatedly observed. Possible explanations are the uptake of genetically different Giardia cysts by a host, or subsequent infection of an already infected host, likely without overt symptoms, with a different Giardia species, which may cause disease. Other explanations for mixed genotypes, particularly for assemblage B, are substantial allelic sequence heterogeneity and/or genetic recombination. Although the zoonotic potential of G. duodenalis is evident, evidence on the contribution and frequency is (still) lacking. This newly developed molecular database has the potential to tackle intricate epidemiological questions concerning protozoan diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
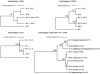
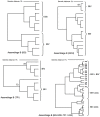
References
-
- Buret AG. Pathophysiology of enteric infections with Giardia duodenalius. Parasite. 2008;15:261–265. - PubMed
-
- Bundy DA, Hall A, Medley GF, Savioli L. Evaluating measures to control intestinal parasitic infections. World Health Stat Q. 1992;45:168–179. - PubMed
-
- Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006;22:203–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

