Virus movements on the plasma membrane support infection and transmission between cells
- PMID: 19956678
- PMCID: PMC2777510
- DOI: 10.1371/journal.ppat.1000621
Virus movements on the plasma membrane support infection and transmission between cells
Abstract
How viruses are transmitted across the mucosal epithelia of the respiratory, digestive, or excretory tracts, and how they spread from cell to cell and cause systemic infections, is incompletely understood. Recent advances from single virus tracking experiments have revealed conserved patterns of virus movements on the plasma membrane, including diffusive motions, drifting motions depending on retrograde flow of actin filaments or actin tail formation by polymerization, and confinement to submicrometer areas. Here, we discuss how viruses take advantage of cellular mechanisms that normally drive the movements of proteins and lipids on the cell surface. A concept emerges where short periods of fast diffusive motions allow viruses to rapidly move over several micrometers. Coupling to actin flow supports directional transport of virus particles during entry and cell-cell transmission, and local confinement coincides with either nonproductive stalling or infectious endocytic uptake. These conserved features of virus-host interactions upstream of infectious entry offer new perspectives for anti-viral interference.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

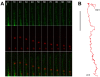

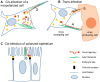
References
-
- Liberali P, Ramo P, Pelkmans L. Protein kinases: starting a molecular-systems view of endocytosis. Annu Rev Cell Dev Biol. 2008;24:501–523. - PubMed
-
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. - PubMed
-
- Greber UF, Way M. A super highway to virus infection. Cell. 2006;124:741–754. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

