PCR-free detection of genetically modified organisms using magnetic capture technology and fluorescence cross-correlation spectroscopy
- PMID: 19956680
- PMCID: PMC2778010
- DOI: 10.1371/journal.pone.0008074
PCR-free detection of genetically modified organisms using magnetic capture technology and fluorescence cross-correlation spectroscopy
Abstract
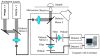
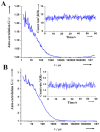
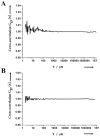
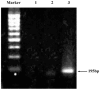
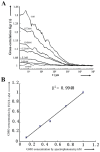
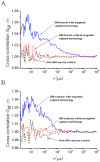
The safety of genetically modified organisms (GMOs) has attracted much attention recently. Polymerase chain reaction (PCR) amplification is a common method used in the identification of GMOs. However, a major disadvantage of PCR is the potential amplification of non-target DNA, causing false-positive identification. Thus, there remains a need for a simple, reliable and ultrasensitive method to identify and quantify GMO in crops. This report is to introduce a magnetic bead-based PCR-free method for rapid detection of GMOs using dual-color fluorescence cross-correlation spectroscopy (FCCS). The cauliflower mosaic virus 35S (CaMV35S) promoter commonly used in transgenic products was targeted. CaMV35S target was captured by a biotin-labeled nucleic acid probe and then purified using streptavidin-coated magnetic beads through biotin-streptavidin linkage. The purified target DNA fragment was hybridized with two nucleic acid probes labeled respectively by Rhodamine Green and Cy5 dyes. Finally, FCCS was used to detect and quantify the target DNA fragment through simultaneously detecting the fluorescence emissions from the two dyes. In our study, GMOs in genetically engineered soybeans and tomatoes were detected, using the magnetic bead-based PCR-free FCCS method. A detection limit of 50 pM GMOs target was achieved and PCR-free detection of GMOs from 5 microg genomic DNA with magnetic capture technology was accomplished. Also, the accuracy of GMO determination by the FCCS method is verified by spectrophotometry at 260 nm using PCR amplified target DNA fragment from GM tomato. The new method is rapid and effective as demonstrated in our experiments and can be easily extended to high-throughput and automatic screening format. We believe that the new magnetic bead-assisted FCCS detection technique will be a useful tool for PCR-free GMOs identification and other specific nucleic acids.
Conflict of interest statement
Figures
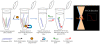
Similar articles
-
Real-time polymerase chain reaction detection of cauliflower mosaic virus to complement the 35S screening assay for genetically modified organisms.J AOAC Int. 2005 May-Jun;88(3):814-22. J AOAC Int. 2005. PMID: 16001857
-
Detection of genetically modified organisms by electrochemiluminescence PCR method.Biosens Bioelectron. 2004 Oct 15;20(3):436-41. doi: 10.1016/j.bios.2004.03.030. Biosens Bioelectron. 2004. PMID: 15494222
-
Detection of nonauthorized genetically modified organisms using differential quantitative polymerase chain reaction: application to 35S in maize.Anal Biochem. 2008 May 15;376(2):189-99. doi: 10.1016/j.ab.2008.02.013. Epub 2008 Feb 21. Anal Biochem. 2008. PMID: 18346452
-
PCR technology for screening and quantification of genetically modified organisms (GMOs).Anal Bioanal Chem. 2003 Apr;375(8):985-93. doi: 10.1007/s00216-003-1767-7. Epub 2003 Feb 15. Anal Bioanal Chem. 2003. PMID: 12733008 Review.
-
New trends in bioanalytical tools for the detection of genetically modified organisms: an update.Anal Bioanal Chem. 2008 Oct;392(3):355-67. doi: 10.1007/s00216-008-2193-7. Epub 2008 Jun 8. Anal Bioanal Chem. 2008. PMID: 18537027 Review.
Cited by
-
Chromatin structure analysis enables detection of DNA insertions into the mammalian nuclear genome.Biochem Biophys Rep. 2015 Jun 10;2:143-152. doi: 10.1016/j.bbrep.2015.06.002. eCollection 2015 Jul. Biochem Biophys Rep. 2015. PMID: 29124156 Free PMC article.
-
Screening for FtsZ Dimerization Inhibitors Using Fluorescence Cross-Correlation Spectroscopy and Surface Resonance Plasmon Analysis.PLoS One. 2015 Jul 8;10(7):e0130933. doi: 10.1371/journal.pone.0130933. eCollection 2015. PLoS One. 2015. PMID: 26154290 Free PMC article.
-
Perspectives on genetically modified crops and food detection.J Food Drug Anal. 2016 Jan;24(1):1-8. doi: 10.1016/j.jfda.2015.06.011. Epub 2015 Jul 30. J Food Drug Anal. 2016. PMID: 28911391 Free PMC article. Review.
References
-
- Gachet E, Martin GG, Vigneau F, Meyer G. Detection of genetically modified organisms(GMOs) by PCR: a brief review of methodologies available. Trends Food Sci Tech. 1999;9:380–388.
-
- Costa-Font M, Gil JM, Traill WB. consumer acceptance, valuation of and attitude towards genetically modified food: review and implications for food policy. Food Policy. 2008;33:99–111.
-
- Council Regulation (EC) No. 49/2000 of the European Parliament and of the Council of 10 January 2000, See Official Journal L006, 11/01/2000, p. 0015.
-
- Ahmed FE. Detection of genetically modified organisms in foods. Trends Biotechnol. 2002;20:215–223. - PubMed
-
- Liu J, Xing D, Shen X, Zhu D. Electrochemiluminescence polymerase chain reaction detection of genetically modified organisms. Biosens Bioelectro. 2005;537:119–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

