The Salmonella SPI2 effector SseI mediates long-term systemic infection by modulating host cell migration
- PMID: 19956712
- PMCID: PMC2777311
- DOI: 10.1371/journal.ppat.1000671
The Salmonella SPI2 effector SseI mediates long-term systemic infection by modulating host cell migration
Abstract
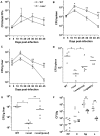
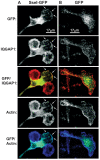
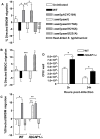
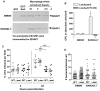
Host-adapted strains of Salmonella enterica cause systemic infections and have the ability to persist systemically for long periods of time despite the presence of a robust immune response. Chronically infected hosts are asymptomatic and transmit disease to naïve hosts via fecal shedding of bacteria, thereby serving as a critical reservoir for disease. We show that the bacterial effector protein SseI (also called SrfH), which is translocated into host cells by the Salmonella Pathogenicity Island 2 (SPI2) type III secretion system (T3SS), is required for Salmonella typhimurium to maintain a long-term chronic systemic infection in mice. SseI inhibits normal cell migration of primary macrophages and dendritic cells (DC) in vitro, and such inhibition requires the host factor IQ motif containing GTPase activating protein 1 (IQGAP1), an important regulator of cell migration. SseI binds directly to IQGAP1 and co-localizes with this factor at the cell periphery. The C-terminal domain of SseI is similar to PMT/ToxA, a bacterial toxin that contains a cysteine residue (C1165) that is critical for activity. Mutation of the corresponding residue in SseI (C178A) eliminates SseI function in vitro and in vivo, but not binding to IQGAP1. In addition, infection with wild-type (WT) S. typhimurium suppressed DC migration to the spleen in vivo in an SseI-dependent manner. Correspondingly, examination of spleens from mice infected with WT S. typhimurium revealed fewer DC and CD4(+) T lymphocytes compared to mice infected with Delta sseI S. typhimurium. Taken together, our results demonstrate that SseI inhibits normal host cell migration, which ultimately counteracts the ability of the host to clear systemic bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–969. - PubMed
-
- Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, et al. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55:585–591. - PubMed
-
- Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. - PubMed
-
- Yen YF, Wang FD, Chiou CS, Chen YY, Lin ML, et al. Prognostic Factors and Clinical Features of Non-typhoid Salmonella Bacteremia in Adults. J Chin Med Assoc. 2009;72:408–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

