Cystatin C is downregulated in prostate cancer and modulates invasion of prostate cancer cells via MAPK/Erk and androgen receptor pathways
- PMID: 19956729
- PMCID: PMC2776515
- DOI: 10.1371/journal.pone.0007953
Cystatin C is downregulated in prostate cancer and modulates invasion of prostate cancer cells via MAPK/Erk and androgen receptor pathways
Abstract
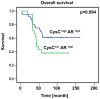
Cystatin C is believed to prevent tumor progression by inhibiting the activities of a family of lysosomal cysteine proteases. However, little is known about the precise mechanism of cystatin C function in prostate cancer. In the present study, we examined the expression of cystatin C and its association with matrix metalloproteinases 2 (MMP2) and androgen receptor (AR) in a tissue microarray comparing benign and malignant specimens from 448 patients who underwent radical prostatectomy for localized prostate cancer. Cystatin C expression was significantly lower in cancer specimens than in benign tissues (p<0.001) and there was a statistically significant inverse correlation between expression of cystatin C and MMP2 (r(s) (2) = -0.056, p = 0.05). There was a clear trend that patients with decreased level of cystatin C had lower overall survival. Targeted inhibition of cystatin C using specific siRNA resulted in an increased invasiveness of PC3 cells, whereas induction of cystatin C overexpression greatly reduced invasion rate of PC3 in vitro. The effect of cystatin C on modulating the PC3 cell invasion was provoked by Erk2 inhibitor that specifically inhibited MAPK/Erk2 activity. This suggests that cystatin C may mediate tumor cell invasion by modulating the activity of MAPK/Erk cascades. Consistent with our immunohistochemical findings that patients with low expression of cystatin C and high expression of androgen receptor (AR) tend to have worse overall survival than patients with high expression of cystatin C and high AR expression, induced overexpression of AR in PC3 cells expressing cystatin C siRNA greatly enhanced the invasiveness of PC3 cells. This suggests that there may be a crosstalk between cystatin C and AR-mediated pathways. Our study uncovers a novel role for cystatin C and its associated cellular pathways in prostate cancer invasion and metastasis.
Conflict of interest statement
Figures






Similar articles
-
Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis.J Natl Cancer Inst. 2008 Jul 16;100(14):1022-36. doi: 10.1093/jnci/djn214. Epub 2008 Jul 8. J Natl Cancer Inst. 2008. PMID: 18612129 Free PMC article.
-
Reduced FRG1 expression promotes prostate cancer progression and affects prostate cancer cell migration and invasion.BMC Cancer. 2019 Apr 11;19(1):346. doi: 10.1186/s12885-019-5509-4. BMC Cancer. 2019. PMID: 30975102 Free PMC article.
-
EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR).Int J Cancer. 2004 Oct 20;112(1):78-86. doi: 10.1002/ijc.20362. Int J Cancer. 2004. PMID: 15305378
-
Androgen receptor and prostate cancer invasion.Int J Androl. 2003 Feb;26(1):21-5. doi: 10.1046/j.1365-2605.2003.00375.x. Int J Androl. 2003. PMID: 12534934 Review.
-
Non-genomic effects of the androgen receptor and vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells.Steroids. 2006 Apr;71(4):304-9. doi: 10.1016/j.steroids.2005.09.010. Epub 2005 Nov 9. Steroids. 2006. PMID: 16289173 Review.
Cited by
-
Externally added cystatin C reduces growth of A375 melanoma cells by increasing cell cycle time.FEBS Open Bio. 2021 Jun;11(6):1645-1658. doi: 10.1002/2211-5463.13162. Epub 2021 May 2. FEBS Open Bio. 2021. PMID: 33837649 Free PMC article.
-
Disruptive environmental chemicals and cellular mechanisms that confer resistance to cell death.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S89-110. doi: 10.1093/carcin/bgv032. Carcinogenesis. 2015. PMID: 26106145 Free PMC article. Review.
-
[Clinical significance of detection of cathepsin X and cystatin C in the sera of patients with lung cancer].Zhongguo Fei Ai Za Zhi. 2013 Aug 20;16(8):411-6. doi: 10.3779/j.issn.1009-3419.2013.08.04. Zhongguo Fei Ai Za Zhi. 2013. PMID: 23945244 Free PMC article. Chinese.
-
Utility of cystatin C as a potential bladder tumour biomarker confirmed by surface plasmon resonance technique.Indian J Med Res. 2018 Jan;147(1):46-50. doi: 10.4103/ijmr.IJMR_124_16. Indian J Med Res. 2018. PMID: 29749360 Free PMC article.
-
Pretreatment Serum Cystatin C Levels Predict Renal Function, but Not Tumor Characteristics, in Patients with Prostate Neoplasia.Biomed Res Int. 2017;2017:7450459. doi: 10.1155/2017/7450459. Epub 2017 Jul 24. Biomed Res Int. 2017. PMID: 28812020 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Coleman RE. Future directions in the treatment and prevention of bone metastases. Am J Clin Oncol. 2002;25:S32–38. - PubMed
-
- Lah TT, Buck MR, Honn KV, Crissman JD, Rao NC, et al. Degradation of laminin by human tumor cathepsin B. Clin Exp Metastasis. 1989;7:461–468. - PubMed
-
- Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–1630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

