Multiple functions of Nm23-H1 are regulated by oxido-reduction system
- PMID: 19956735
- PMCID: PMC2776532
- DOI: 10.1371/journal.pone.0007949
Multiple functions of Nm23-H1 are regulated by oxido-reduction system
Abstract
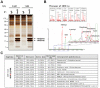
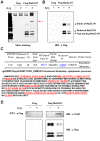
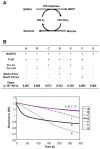
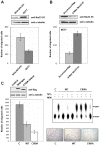
Nucleoside diphosphate kinase (NDPK, Nm23), a housekeeping enzyme, is known to be a multifunctional protein, acting as a metastasis suppressor, transactivation activity on c-myc, and regulating endocytosis. The cellular mechanisms regulating Nm23 functions are poorly understood. In this study, we identified the modifications and interacting proteins of Nm23-H1 in response to oxidative stress. We found that Cys109 in Nm23-H1 is oxidized to various oxidation states including intra- and inter-disulfide crosslinks, glutathionylation, and sulfonic acid formation in response to H(2)O(2) treatment both in vivo and in vitro. The cross-linking sites and modifications of oxidized Nm23-H1 were identified by peptide sequencing using UPLC-ESI-q-TOF tandem MS. Glutathionylation and oxidation of Cys109 inhibited the NDPK enzymatic activity of Nm23-H1. We also found that thioredoxin reductase 1 (TrxR1) is an interacting protein of Nm23-H1, and it binds specifically to oxidized Nm23-H1. Oxidized Nm23 is a substrate of NADPH-TrxR1-thioredoxin shuttle system, and the disulfide crosslinking is reversibly reduced and the enzymatic activity is recovered by this system. Oxidation of Cys109 in Nm23-H1 inhibited its metastatic suppressor activity as well as the enzymatic activities. The mutant, Nm23-H1 C109A, retained both the enzymatic and metastasis suppressor activities under oxidative stress. This suggests that key enzymatic and metastasis suppressor functions of Nm23-H1 are regulated by oxido-reduction of its Cys109.
Conflict of interest statement
Figures




References
-
- Rosengard AM, Krutzsch HC, Shearn A, Biggs JR, Barker E, et al. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989;342:177–180. - PubMed
-
- Nakayama T, Ohtsuru A, Nakao K, Shima M, Nakata K, et al. Expression in human hepatocellular carcinoma of nucleoside diphosphate kinase, a homologue of the nm23 gene product. J Natl Cancer Inst. 1992;84:1349–1354. - PubMed
-
- Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988;80:200–204. - PubMed
-
- Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. - PubMed
-
- Leone A, Flatow U, VanHoutte K, Steeg PS. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene. 1993;8:2325–2333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

