Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus
- PMID: 19956766
- PMCID: PMC2776306
- DOI: 10.1371/journal.pgen.1000739
Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus
Abstract
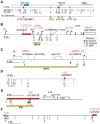
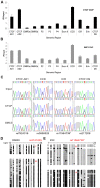
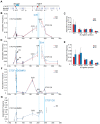
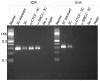
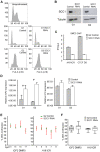
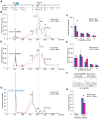
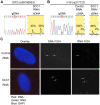
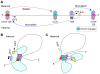
Cohesin is a chromatin-associated protein complex that mediates sister chromatid cohesion by connecting replicated DNA molecules. Cohesin also has important roles in gene regulation, but the mechanistic basis of this function is poorly understood. In mammalian genomes, cohesin co-localizes with CCCTC binding factor (CTCF), a zinc finger protein implicated in multiple gene regulatory events. At the imprinted IGF2-H19 locus, CTCF plays an important role in organizing allele-specific higher-order chromatin conformation and functions as an enhancer blocking transcriptional insulator. Here we have used chromosome conformation capture (3C) assays and RNAi-mediated depletion of cohesin to address whether cohesin affects higher order chromatin conformation at the IGF2-H19 locus in human cells. Our data show that cohesin has a critical role in maintaining CTCF-mediated chromatin conformation at the locus and that disruption of this conformation coincides with changes in IGF2 expression. We show that the cohesin-dependent, higher-order chromatin conformation of the locus exists in both G1 and G2 phases of the cell cycle and is therefore independent of cohesin's function in sister chromatid cohesion. We propose that cohesin can mediate interactions between DNA molecules in cis to insulate genes through the formation of chromatin loops, analogous to the cohesin mediated interaction with sister chromatids in trans to establish cohesion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

