Characterization of mucosal Candida albicans biofilms
- PMID: 19956771
- PMCID: PMC2776351
- DOI: 10.1371/journal.pone.0007967
Characterization of mucosal Candida albicans biofilms
Abstract
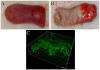
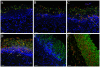
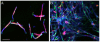
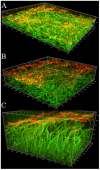
C. albicans triggers recurrent infections of the alimentary tract mucosa that result from biofilm growth. Although the ability of C. albicans to form a biofilm on abiotic surfaces has been well documented in recent years, no information exists on biofilms that form directly on mucosal surfaces. The objectives of this study were to characterize the structure and composition of Candida biofilms forming on the oral mucosa. We found that oral Candida biofilms consist of yeast, hyphae, and commensal bacteria, with keratin dispersed in the intercellular spaces. Neutrophils migrate through the oral mucosa and form nests within the biofilm mass. The cell wall polysaccharide beta-glucan is exposed during mucosal biofilm growth and is more uniformly present on the surface of biofilm organisms invading the oral mucosa. We conclude that C. albicans forms complex mucosal biofilms consisting of both commensal bacterial flora and host components. These discoveries are important since they can prompt a shift of focus for current research in investigating the role of Candida-bacterial interactions in the pathogenesis of mucosal infections as well as the role of beta-glucan mediated signaling in the host response.
Conflict of interest statement
Figures



References
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. - PubMed
-
- Post JC, Hiller NL, Nistico L, Stoodley P, Ehrlich GD. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15:347–351. - PubMed
-
- Odds FC. Activity of Cilofungin (Ly121019) against Candida species invitro. J Antimicrobial Chem. 1988;22:891–897. - PubMed
-
- Seneviratne CJ, Jin L, Samaranayake LP. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008;14:582–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

