Influence of "Flexible" versus "Rigid" Nanoparticles on the Stability of Matrix Metalloproteinase-7
- PMID: 19956790
- PMCID: PMC2759757
- DOI: 10.1166/jbn.2008.010
Influence of "Flexible" versus "Rigid" Nanoparticles on the Stability of Matrix Metalloproteinase-7
Abstract
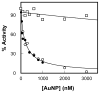
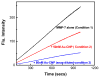
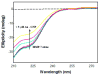
Matrix Metalloproteinase-7 (MMP-7) is invariably expressed in a variety of cancer cells, and exhibits the potentials to interact with differently charged macromolecular surfaces 1. To ascertain whether the nature of the charge carrying surfaces influences the stability as well as catalytic properties of the enzyme, we compared the effects of differently charged lipid (representative of "flexible") and gold ("rigid") nanoparticles. The experimental data revealed that the catalytic activity of MMP-7 is impaired only by the positively charged lipid nanoparticles, and it remains unaffected by their negatively charged or neutral counterparts. On the other hand, both positively and negatively charged gold nanoparticles impair the enzyme activity with nearly equal potency; no significant influence of neutral gold nanoparticles was noted on the enzyme activity. Unlike lipid nanoparticles, the charged gold nanoparticles mediated effects were found to be manifested partially via the inactivation of the enzyme. Arguments are presented that both the "rigidity" as well as the surface curvature of the lipid ("flexible") vis a vis the gold ("rigid") nanoparticles are responsible for eliciting differential influence on the catalytic activity as well as the stability of MMP-7.
Figures


References
-
- Overall CM, Kleifeld O. Tumor microenvironment- opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. - PubMed
-
- Davenport EL, Morgan GJ, Davies FE. Untangling the unfolded protein response. Cell Cycle. 2008;7(7):865–869. - PubMed
-
- Bhattacharya R, Mukherjee P. Biological properties of “naked” nanoparticles. Adv Drug Deliv Rev. 2008 (in press) - PubMed
-
- Korolev N, Vorontsova OV, Nordenskiold L. Physicochemical analysis of electrostatic foundation for DNA-protein interactions in chromatin transformations. Prog Biophys Mol Biol, Biopolymers. 2007;88(3):325–339. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
