Identification of positive regulators of the yeast fps1 glycerol channel
- PMID: 19956799
- PMCID: PMC2773846
- DOI: 10.1371/journal.pgen.1000738
Identification of positive regulators of the yeast fps1 glycerol channel
Abstract
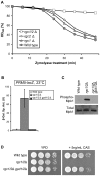
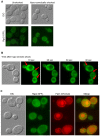
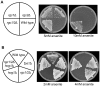
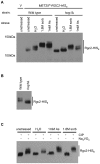
The yeast Fps1 protein is an aquaglyceroporin that functions as the major facilitator of glycerol transport in response to changes in extracellular osmolarity. Although the High Osmolarity Glycerol pathway is thought to have a function in at least basal control of Fps1 activity, its mode of regulation is not understood. We describe the identification of a pair of positive regulators of the Fps1 glycerol channel, Rgc1 (Ypr115w) and Rgc2 (Ask10). An rgc1/2Delta mutant experiences cell wall stress that results from osmotic pressure associated with hyper-accumulation of glycerol. Accumulation of glycerol in the rgc1/2Delta mutant results from a defect in Fps1 activity as evidenced by suppression of the defect through Fps1 overexpression, failure to release glycerol upon hypo-osmotic shock, and resistance to arsenite, a toxic metalloid that enters the cell through Fps1. Regulation of Fps1 by Rgc1/2 appears to be indirect; however, evidence is presented supporting the view that Rgc1/2 regulate Fps1 channel activity, rather than its expression, folding, or localization. Rgc2 was phosphorylated in response to stresses that lead to regulation of Fps1. This stress-induced phosphorylation was partially dependent on the Hog1 MAPK. Hog1 was also required for basal phosphorylation of Rgc2, suggesting a mechanism by which Hog1 may regulate Fps1 indirectly.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Nevoigt E, Stahl U. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Micro Rev. 1997;21:231–241. - PubMed
-
- Kayingo G, Wong B. The MAP kinase Hog1p differentially regulates stress-induced production and accumulation of glycerol and D-arabitol in Candida albicans. Microbiology. 2005;151:2987–2999. - PubMed
-
- Tamas MJ, Luyten K, Sutherland FCW, Hernandez A, Albertyn J, et al. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Molec Micro. 1999;31:1087–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

