A practical guide to rodent islet isolation and assessment
- PMID: 19957062
- PMCID: PMC3056052
- DOI: 10.1007/s12575-009-9021-0
A practical guide to rodent islet isolation and assessment
Abstract
Pancreatic islets of Langerhans secrete hormones that are vital to the regulation of blood glucose and are, therefore, a key focus of diabetes research. Purifying viable and functional islets from the pancreas for study is an intricate process. This review highlights the key elements involved with mouse and rat islet isolation, including choices of collagenase, the collagenase digestion process, purification of islets using a density gradient, and islet culture conditions. In addition, this paper reviews commonly used techniques for assessing islet viability and function, including visual assessment, fluorescent markers of cell death, glucose-stimulated insulin secretion, and intracellular calcium measurements. A detailed protocol is also included that describes a common method for rodent islet isolation that our laboratory uses to obtain viable and functional mouse islets for in vitro study of islet function, beta-cell physiology, and in vivo rodent islet transplantation. The purpose of this review is to serve as a resource and foundation for successfully procuring and purifying high-quality islets for research purposes.
Figures
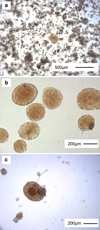

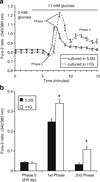


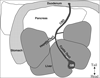

References
-
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16(1):35–39. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

