Peloruside B, a potent antitumor macrolide from the New Zealand marine sponge Mycale hentscheli: isolation, structure, total synthesis, and bioactivity
- PMID: 19957922
- PMCID: PMC2812918
- DOI: 10.1021/jo9021265
Peloruside B, a potent antitumor macrolide from the New Zealand marine sponge Mycale hentscheli: isolation, structure, total synthesis, and bioactivity
Abstract
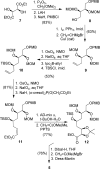
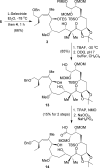
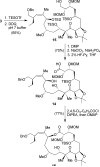
Peloruside B (2), a natural congener of peloruside A (1), was isolated in sub-milligram quantities from the New Zealand marine sponge Mycale hentscheli. Peloruside B promotes microtubule polymerization and arrests cells in the G(2)/M phase of mitosis similar to paclitaxel, and its bioactivity was comparable to that of peloruside A. NMR-directed isolation, structure elucidation, structure confirmation by total synthesis, and bioactivity of peloruside B are described in this article. The synthesis features Sharpless dihydroxylation, Brown's asymmetric allylboration reaction, reductive aldol coupling, Yamaguchi macrolactonization, and selective methylation.
Figures






Similar articles
-
Microtubule-stabilizing drugs from marine sponges: focus on peloruside A and zampanolide.Mar Drugs. 2010 Mar 31;8(4):1059-79. doi: 10.3390/md8041059. Mar Drugs. 2010. PMID: 20479967 Free PMC article. Review.
-
Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule- stabilizing activity.Cancer Res. 2002 Jun 15;62(12):3356-60. Cancer Res. 2002. PMID: 12067973
-
Structure-activity studies of the pelorusides: new congeners and semi-synthetic analogues.Org Biomol Chem. 2011 Jun 21;9(12):4456-66. doi: 10.1039/c0ob01127d. Epub 2011 Apr 21. Org Biomol Chem. 2011. PMID: 21512693
-
Peloruside A: a potent cytotoxic macrolide isolated from the new zealand marine sponge Mycale sp.J Org Chem. 2000 Jan 28;65(2):445-9. doi: 10.1021/jo991296y. J Org Chem. 2000. PMID: 10813954
-
Peloruside A: a lead non-taxoid-site microtubule-stabilizing agent with potential activity against cancer, neurodegeneration, and autoimmune disease.Nat Prod Rep. 2016 Apr;33(4):549-61. doi: 10.1039/c5np00146c. Epub 2016 Feb 12. Nat Prod Rep. 2016. PMID: 26867978 Review.
Cited by
-
Recent progress with microtubule stabilizers: new compounds, binding modes and cellular activities.Nat Prod Rep. 2014 Mar;31(3):335-55. doi: 10.1039/c3np70092e. Nat Prod Rep. 2014. PMID: 24481420 Free PMC article. Review.
-
Chemistry and Biological Activities of the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria.Mar Drugs. 2018 Jun 18;16(6):214. doi: 10.3390/md16060214. Mar Drugs. 2018. PMID: 29912171 Free PMC article. Review.
-
Microtubule-stabilizing drugs from marine sponges: focus on peloruside A and zampanolide.Mar Drugs. 2010 Mar 31;8(4):1059-79. doi: 10.3390/md8041059. Mar Drugs. 2010. PMID: 20479967 Free PMC article. Review.
-
First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case.Mar Drugs. 2018 Feb 20;16(2):68. doi: 10.3390/md16020068. Mar Drugs. 2018. PMID: 29461501 Free PMC article.
-
The assembly-inducing laulimalide/peloruside a binding site on tubulin: molecular modeling and biochemical studies with [³H]peloruside A.J Chem Inf Model. 2010 Nov 22;50(11):2019-28. doi: 10.1021/ci1002894. Epub 2010 Oct 28. J Chem Inf Model. 2010. PMID: 21028850 Free PMC article.
References
-
- Perry NB, Blunt JW, Munro MHG. J. Am. Chem. Soc. 1988;110:4851–4853.
-
- Perry NB, Blunt JW, Munro MHG, Thompson AM. J. Org. Chem. 1990;55:223–227.
-
- West LM, Northcote PT, Hood KA, Miller JH, Page MJ. J. Nat. Prod. 2000;63:707–709. - PubMed
-
- Northcote PT, Blunt JW, Munro MHG. Tetrahedron Lett. 1991;32:6411–6414.
-
- Burres NS, Clement JJ. Cancer Res. 1989;49:2935–2940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

