Enhancing the intestinal absorption of molecules containing the polar guanidino functionality: a double-targeted prodrug approach
- PMID: 19957998
- PMCID: PMC3304101
- DOI: 10.1021/jm9011559
Enhancing the intestinal absorption of molecules containing the polar guanidino functionality: a double-targeted prodrug approach
Abstract
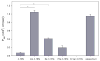
A prodrug strategy was applied to guanidino-containing analogues to increase oral absorption via hPEPT1 and hVACVase. l-Valine, l-isoleucine, and l-phenylalanine esters of [3-(hydroxymethyl)phenyl]guanidine (3-HPG) were synthesized and evaluated for transport and activation. In HeLa/hPEPT1 cells, Val-3-HPG and Ile-3-HPG exhibited high affinity to hPEPT1 (IC(50): 0.65 and 0.63 mM, respectively), and all three l-amino acid esters showed higher uptake (2.6- to 9-fold) than the parent compound 3-HPG. Val-3-HPG and Ile-3-HPG demonstrated remarkable Caco-2 permeability enhancement, and Val-3-HPG exhibited comparable permeability to valacyclovir. In rat perfusion studies, Val-3-HPG and Ile-3-HPG permeabilities were significantly higher than 3-HPG and exceeded/matched the high-permeability standard metoprolol, respectively. All the l-amino acid 3-HPG esters were effectively activated in HeLa and Caco-2 cell homogenates and were found to be good substrates of hVACVase (k(cat)/K(m) in mM(-1) x s(-1): Val-3-HPG, 3370; Ile-3-HPG, 1580; Phe-3-HPG, 1660). In conclusion, a prodrug strategy is effective at increasing the intestinal permeability of polar guanidino analogues via targeting hPEPT1 for transport and hVACVase for activation.
Figures



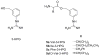
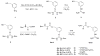
Similar articles
-
Increasing oral absorption of polar neuraminidase inhibitors: a prodrug transporter approach applied to oseltamivir analogue.Mol Pharm. 2013 Feb 4;10(2):512-22. doi: 10.1021/mp300564v. Epub 2013 Jan 4. Mol Pharm. 2013. PMID: 23244438 Free PMC article.
-
Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter.Pharm Res. 1998 Sep;15(9):1382-6. doi: 10.1023/a:1011945420235. Pharm Res. 1998. PMID: 9755889
-
5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter.Pharm Res. 1998 Aug;15(8):1154-9. doi: 10.1023/a:1011919319810. Pharm Res. 1998. PMID: 9706043
-
Mechanistic enhancement of the intestinal absorption of drugs containing the polar guanidino functionality.Expert Opin Drug Metab Toxicol. 2011 Mar;7(3):313-23. doi: 10.1517/17425255.2011.550875. Epub 2011 Jan 14. Expert Opin Drug Metab Toxicol. 2011. PMID: 21235283 Review.
-
Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting.J Pharm Sci. 2008 Mar;97(3):1109-34. doi: 10.1002/jps.21047. J Pharm Sci. 2008. PMID: 17696166 Review.
Cited by
-
The rationality for using prodrug approach in drug discovery programs for new xenobiotics: opportunities and challenges.Eur J Drug Metab Pharmacokinet. 2011 Jun;36(2):49-59. doi: 10.1007/s13318-011-0035-z. Epub 2011 Mar 15. Eur J Drug Metab Pharmacokinet. 2011. PMID: 21404122 Review.
-
Provisional in-silico biopharmaceutics classification (BCS) to guide oral drug product development.Drug Des Devel Ther. 2014 Sep 24;8:1563-75. doi: 10.2147/DDDT.S68909. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25284986 Free PMC article.
-
Hydrotropic Solubilization of Lipophilic Drugs for Oral Delivery: The Effects of Urea and Nicotinamide on Carbamazepine Solubility-Permeability Interplay.Front Pharmacol. 2016 Oct 25;7:379. doi: 10.3389/fphar.2016.00379. eCollection 2016. Front Pharmacol. 2016. PMID: 27826241 Free PMC article.
-
Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice.Drug Metab Dispos. 2013 Mar;41(3):608-14. doi: 10.1124/dmd.112.049239. Epub 2012 Dec 21. Drug Metab Dispos. 2013. PMID: 23264448 Free PMC article.
-
Modern prodrug design for targeted oral drug delivery.Molecules. 2014 Oct 14;19(10):16489-505. doi: 10.3390/molecules191016489. Molecules. 2014. PMID: 25317578 Free PMC article.
References
-
- Ettmayer P, Amidon GL, Clement B, Testa B. Lessons learned from marketed and investigational prodrugs. J Med Chem. 2004;47 (10):2393–2404. - PubMed
-
- Brandsch M, Knutter I, Leibach FH. The intestinal H+/peptide symporter PEPT1: structure-affinity relationships. Eur J Pharm Sci. 2004;21(1):53–60. - PubMed
-
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447(5):610–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

