Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study
- PMID: 19958527
- PMCID: PMC2794841
- DOI: 10.1186/1471-2466-9-49
Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study
Abstract
Background: Despite consensus criteria, diagnosing acute lung injury, or its more severe form acute respiratory distress syndrome (ALI/ARDS) remains challenging. Adding objective measures, such as plasma levels of biological markers could facilitate recognition of ALI/ARDS. This study was designed to assess and compare the diagnostic accuracy of biological markers for ALI/ARDS with ventilator-associated pneumonia (VAP).
Methods: We performed serial measurements of Clara cell protein (CC16), soluble receptor for advanced glycation end products (sRAGE), surfactant protein D (SP-D) and Krebs von den Lungen (KL-6) in plasma of patients with VAP and mechanically ventilated control patients without VAP. ALI/ARDS was diagnosed using the criteria of the North-American European consensus conference.
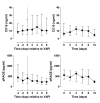
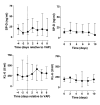
Results: Thirty-seven patients were enrolled - 22 patients with VAP and 15 control patients. Ten patients with pneumonia met the ALI/ARDS consensus criteria. Control patients never met these criteria. Plasma CC16 had a good diagnostic capacity for ALI/ARDS as shown by the receiver operating characteristic curve with an area under the curve of 0.91 (95% confidence interval (CI) 0.79 - 1.00; p < 0.001). Identification of ALI/ARDS patients by sudden increases in plasma CC16 of 30% or more yielded a sensitivity of 90% and a specificity of 92%. Of note, levels of CC16 increased 2 days before ALI/ARDS diagnosis. A cut-off level of 50 ng/ml SP-D yielded a specificity of 100% while the sensitivity was 70%. The area under the curve for SP-D was 0.80 (95% CI 0.58 - 1.00; p = 0.02). The diagnostic accuracies of KL-6 and sRAGE were low.
Conclusion: Plasma CC16 seems a potential biological marker for ALI/ARDS in patients with VAP. Plasma levels of sRAGE, SP-D and KL-6 have limited discriminative power for diagnosing ALI/ARDS in VAP.
Figures



References
-
- Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand J-A, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F. for the ALIVE Study Group. Epidemiology and outcome of acute lung injury in European intensive care units: Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. - DOI - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

