A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction
- PMID: 19958962
- PMCID: PMC3580848
- DOI: 10.1016/j.jacc.2009.06.055
A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction
Abstract
Objectives: Our aim was to investigate the safety and efficacy of intravenous allogeneic human mesenchymal stem cells (hMSCs) in patients with myocardial infarction (MI).
Background: Bone marrow-derived hMSCs may ameliorate consequences of MI, and have the advantages of preparation ease, allogeneic use due to immunoprivilege, capacity to home to injured tissue, and extensive pre-clinical support.
Methods: We performed a double-blind, placebo-controlled, dose-ranging (0.5, 1.6, and 5 million cells/kg) safety trial of intravenous allogeneic hMSCs (Prochymal, Osiris Therapeutics, Inc., Baltimore, Maryland) in reperfused MI patients (n=53). The primary end point was incidence of treatment-emergent adverse events within 6 months. Ejection fraction and left ventricular volumes determined by echocardiography and magnetic resonance imaging were exploratory efficacy end points.
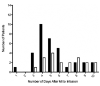
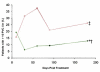
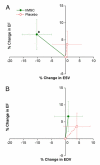
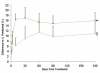
Results: Adverse event rates were similar between the hMSC-treated (5.3 per patient) and placebo-treated (7.0 per patient) groups, and renal, hepatic, and hematologic laboratory indexes were not different. Ambulatory electrocardiogram monitoring demonstrated reduced ventricular tachycardia episodes (p=0.025), and pulmonary function testing demonstrated improved forced expiratory volume in 1 s (p=0.003) in the hMSC-treated patients. Global symptom score in all patients (p=0.027) and ejection fraction in the important subset of anterior MI patients were both significantly better in hMSCs versus placebo subjects. In the cardiac magnetic resonance imaging substudy, hMSC treatment, but not placebo, increased left ventricular ejection fraction and led to reverse remodeling.
Conclusions: Intravenous allogeneic hMSCs are safe in patients after acute MI. This trial provides pivotal safety and provisional efficacy data for an allogeneic bone marrow-derived stem cell in post-infarction patients. (Safety Study of Adult Mesenchymal Stem Cells [MSC] to Treat Acute Myocardial Infarction; NCT00114452).
Figures


Comment in
-
From mice to men. Commonalities in physiology for stem cell-based cardiac repair.J Am Coll Cardiol. 2009 Dec 8;54(24):2287-9. doi: 10.1016/j.jacc.2009.06.054. J Am Coll Cardiol. 2009. PMID: 19958963 No abstract available.
References
-
- Boyle AJ, Schulman SP, Hare JM. Is stem cell therapy ready for patients? Stem cell therapy for cardiac repair—ready for the next step. Circulation. 2006;114:339–52. - PubMed
-
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. - PubMed
-
- Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. - PubMed
-
- Dill T, Schachinger V, Rolf A, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–7. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

