Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein
- PMID: 19959470
- PMCID: PMC2823541
- DOI: 10.1074/jbc.M109.034421
Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein
Abstract
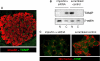
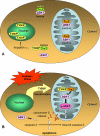
The thioredoxin-interacting protein TXNIP is a ubiquitously expressed redox protein that promotes apoptosis. Recently, we found that TXNIP deficiency protects against type 1 and 2 diabetes by inhibiting beta cell apoptosis and maintaining pancreatic beta cell mass, indicating that TXNIP plays a key role in beta cell biology. However, very little is known about the intracellular localization and function of TXNIP, and although TXNIP has been thought to be a cytoplasmic protein, our immunohistochemistry studies in beta cells surprisingly revealed a nuclear TXNIP localization, suggesting that TXNIP may shuttle within the cell. Using immunohistochemistry/confocal imaging and cell fractionation/co-immunoprecipitation, we found that, under physiological conditions, TXNIP is localized primarily in the nucleus of pancreatic beta cells, whereas oxidative stress leads to TXNIP shuttling into the mitochondria. In mitochondria, TXNIP binds to and oxidizes Trx2, thereby reducing Trx2 binding to ASK1 and allowing for ASK1 phosphorylation/activation, resulting in induction of the mitochondrial pathway of apoptosis with cytochrome c release and caspase-3 cleavage. TXNIP overexpression and Trx2 (but not cytosolic Trx1) silencing mimic these effects. Thus, we discovered that TXNIP shuttles between subcellular compartments in response to oxidative stress and identified a novel redox-sensitive mitochondrial TXNIP-Trx2-ASK1 signaling cascade.
Figures






Similar articles
-
Blocking CHOP-dependent TXNIP shuttling to mitochondria attenuates albuminuria and mitigates kidney injury in nephrotic syndrome.Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2116505119. doi: 10.1073/pnas.2116505119. Epub 2022 Aug 22. Proc Natl Acad Sci U S A. 2022. PMID: 35994650 Free PMC article.
-
Age-related changes in mitochondrial antioxidant enzyme Trx2 and TXNIP-Trx2-ASK1 signal pathways in the auditory cortex of a mimetic aging rat model: changes to Trx2 in the auditory cortex.FEBS J. 2015 Jul;282(14):2758-74. doi: 10.1111/febs.13324. Epub 2015 Jun 10. FEBS J. 2015. PMID: 25996168
-
Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner.Circ Res. 2004 Jun 11;94(11):1483-91. doi: 10.1161/01.RES.0000130525.37646.a7. Epub 2004 Apr 29. Circ Res. 2004. PMID: 15117824
-
Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer.Mitochondrion. 2013 May;13(3):163-9. doi: 10.1016/j.mito.2012.06.004. Epub 2012 Jun 27. Mitochondrion. 2013. PMID: 22750447 Review.
-
Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic β-cell.Mol Endocrinol. 2014 Aug;28(8):1211-20. doi: 10.1210/me.2014-1095. Epub 2014 Jun 9. Mol Endocrinol. 2014. PMID: 24911120 Free PMC article. Review.
Cited by
-
Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells.J Biol Chem. 2013 Mar 8;288(10):6835-48. doi: 10.1074/jbc.M112.419101. Epub 2013 Jan 17. J Biol Chem. 2013. PMID: 23329835 Free PMC article.
-
The cooperation between the autophagy machinery and the inflammasome to implement an appropriate innate immune response: do they regulate each other?Immunol Rev. 2015 May;265(1):194-204. doi: 10.1111/imr.12288. Immunol Rev. 2015. PMID: 25879294 Free PMC article. Review.
-
The Role of Txnip in Mitophagy Dysregulation and Inflammasome Activation in Diabetic Retinopathy: A New Perspective.JOJ Ophthalmol. 2017;4(4):10.19080/jojo.2017.04.555643. doi: 10.19080/jojo.2017.04.555643. Epub 2017 Sep 15. JOJ Ophthalmol. 2017. PMID: 29376145 Free PMC article.
-
Thioredoxin-interacting protein mediates dysfunction of tubular autophagy in diabetic kidneys through inhibiting autophagic flux.Lab Invest. 2014 Mar;94(3):309-20. doi: 10.1038/labinvest.2014.2. Epub 2014 Feb 3. Lab Invest. 2014. PMID: 24492284
-
Mitochondria-associated membranes (MAMs) and inflammation.Cell Death Dis. 2018 Feb 28;9(3):329. doi: 10.1038/s41419-017-0027-2. Cell Death Dis. 2018. PMID: 29491386 Free PMC article. Review.
References
-
- Chen K. S., DeLuca H. F. (1994) Biochim. Biophys. Acta 1219, 26–32 - PubMed
-
- Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. (1999) J. Biol. Chem. 274, 21645–21650 - PubMed
-
- Junn E., Han S. H., Im J. Y., Yang Y., Cho E. W., Um H. D., Kim D. K., Lee K. W., Han P. L., Rhee S. G., Choi I. (2000) J. Immunol. 164, 6287–6295 - PubMed
-
- Yamanaka H., Maehira F., Oshiro M., Asato T., Yanagawa Y., Takei H., Nakashima Y. (2000) Biochem. Biophys. Res. Commun. 271, 796–800 - PubMed
-
- Nishiyama A., Masutani H., Nakamura H., Nishinaka Y., Yodoi J. (2001) IUBMB Life 52, 29–33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

