Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database
- PMID: 19959591
- PMCID: PMC2788912
- DOI: 10.1136/bmj.b4731
Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database
Abstract
Objective: To investigate the risk of incident myocardial infarction, congestive heart failure, and all cause mortality associated with prescription of oral antidiabetes drugs.
Design: Retrospective cohort study.
Setting: UK general practice research database, 1990-2005.
Participants: 91,521 people with diabetes.
Main outcome measures: Incident myocardial infarction, congestive heart failure, and all cause mortality. Person time intervals for drug treatment were categorised by drug class, excluding non-drug intervals and intervals for insulin.
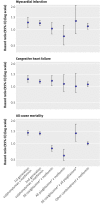
Results: 3588 incident cases of myocardial infarction, 6900 of congestive heart failure, and 18,548 deaths occurred. Compared with metformin, monotherapy with first or second generation sulphonylureas was associated with a significant 24% to 61% excess risk for all cause mortality (P<0.001) and second generation sulphonylureas with an 18% to 30% excess risk for congestive heart failure (P=0.01 and P<0.001). The thiazolidinediones were not associated with risk of myocardial infarction; pioglitazone was associated with a significant 31% to 39% lower risk of all cause mortality (P=0.02 to P<0.001) compared with metformin. Among the thiazolidinediones, rosiglitazone was associated with a 34% to 41% higher risk of all cause mortality (P=0.14 to P=0.01) compared with pioglitazone. A large number of potential confounders were accounted for in the study; however, the possibility of residual confounding or confounding by indication (differences in prognostic factors between drug groups) cannot be excluded.
Conclusions: Our findings suggest a relatively unfavourable risk profile of sulphonylureas compared with metformin for all outcomes examined. Pioglitazone was associated with reduced all cause mortality compared with metformin. Pioglitazone also had a favourable risk profile compared with rosiglitazone; although this requires replication in other studies, it may have implications for prescribing within this class of drugs.
Conflict of interest statement
Competing interests: PE is a coprincipal investigator on a grant cofunded by the UK Medical Research Council and GlaxoSmithKline. MRW has received consultancy fees from GlaxoSmithKline in the past five years. MM has received grants from Pfizer, AstraZeneca, and the Serious Adverse Events Consortium (collaboration between industry and academia). KK has acted in a consultant capacity or as a speaker for Novo-Nordisk, Sanofi, Lilli, Merck Sharp & Dohme, Tekeda, GSK, and Bayer and has received research grants from Servier, Novartis, Novo-Nordisk, Sanofi-Aventis, Merck Sharp & Dohme, Pfizer, Bayer, Unilever, and Lilly.
Figures
References
-
- World Health Organization. Diabetes. 2008. www.who.int/mediacentre/factsheets/fs312/en/index.html.
-
- Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129-36. - PubMed
-
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457-71. - PubMed
-
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125-35. - PubMed
-
- Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 2007;147:578-81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
