Ca2+ dysregulation in Ryr1(I4895T/wt) mice causes congenital myopathy with progressive formation of minicores, cores, and nemaline rods
- PMID: 19959667
- PMCID: PMC2788482
- DOI: 10.1073/pnas.0912126106
Ca2+ dysregulation in Ryr1(I4895T/wt) mice causes congenital myopathy with progressive formation of minicores, cores, and nemaline rods
Abstract
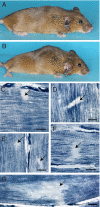
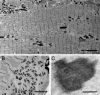
Ryr1(I4895T/wt) (IT/+) mice express a knockin mutation corresponding to the human I4898T EC-uncoupling mutation in the type 1 ryanodine receptor/Ca(2+) release channel (RyR1), which causes a severe form of central core disease (CCD). IT/+ mice exhibit a slowly progressive congenital myopathy, with neonatal respiratory stress, skeletal muscle weakness, impaired mobility, dorsal kyphosis, and hind limb paralysis. Lesions observed in myofibers from diseased mice undergo age-dependent transformation from minicores to cores and nemaline rods. Early ultrastructural abnormalities include sarcomeric misalignment, Z-line streaming, focal loss of cross-striations, and myofibrillar splitting and intermingling that may arise from defective myofibrillogenesis. However, manifestation of the disease phenotype is highly variable on a Sv129 genomic background. Quantitative RT-PCR shows an equimolar ratio of WT and mutant Ryr1 transcripts within IT/+ myofibers and total RyR1 protein expression levels are normal. We propose a unifying theory in which the cause of core formation lies in functional heterogeneity among RyR1 tetramers. Random combinations of normal and either leaky or EC-uncoupled RyR subunits would lead to spatial differences in Ca(2+) transients; the resulting heterogeneity of contraction among myofibrils would lead to focal, irreversible tearing and shearing, which would, over time, enlarge to form minicores, cores, and nemaline rods. The IT/+ mouse line is proposed to be a valid model of RyR1-related congenital myopathy, offering high potential for elucidation of the pathogenesis of skeletal muscle disorders arising from impaired EC coupling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sharma MC, Jain D, Sarkar C, Goebel HH. Congenital myopathies–a comprehensive update of recent advancements. Acta Neurol Scand. 2009;119:281–292. - PubMed
-
- Sewry CA, Jimenez-Mallebrera C, Muntoni F. Congenital myopathies. Curr Opin Neurol. 2008;21:569–575. - PubMed
-
- Dubowitz V, Sewry CA. Muscle Biopsy: A Practical Approach. 3rd Edition. Amsterdam: Elsevier; 2007.
-
- Bethlem J, Arts WF, Dingemans KP. Common origin of rods, cores, miniature cores, and focal loss of cross-striations. Arch Neurol. 1978;35:555–566. - PubMed
-
- Vallat JM, et al. Coexistence of minicores, cores, and rods in the same muscle biopsy. A new example of mixed congenital myopathy. Acta Neuropathol. 1982;58:229–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

