Small cytoskeleton-associated molecule, fibroblast growth factor receptor 1 oncogene partner 2/wound inducible transcript-3.0 (FGFR1OP2/wit3.0), facilitates fibroblast-driven wound closure
- PMID: 19959814
- PMCID: PMC2797874
- DOI: 10.2353/ajpath.2010.090256
Small cytoskeleton-associated molecule, fibroblast growth factor receptor 1 oncogene partner 2/wound inducible transcript-3.0 (FGFR1OP2/wit3.0), facilitates fibroblast-driven wound closure
Abstract
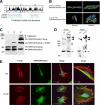
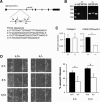
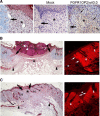
Wounds created in the oral cavity heal rapidly and leave minimal scarring. We have examined a role of a previously isolated cDNA from oral wounds encoding wound inducible transcript-3.0 (wit3.0), also known as fibroblast growth factor receptor 1 oncogene partner 2 (FGFR1OP2). FGFR1OP2/wit3.0 was highly expressed in oral wound fibroblasts without noticeable up-regulation of alpha-smooth muscle actin. In silico analyses, denaturing and nondenaturing gel Western blot, and immunocytology together demonstrated that FGFR1OP2/wit3.0 were able to dimerize and oligomerize through coiled-coil structures and appeared to associate with cytoskeleton networks in oral wound fibroblasts. Overexpression of FGFR1OP2/wit3.0 increased the floating collagen gel contraction of naïve oral fibroblasts to the level of oral wound fibroblasts, which was in turn attenuated by small-interfering RNA knockdown. The FGFR1OP2/wit3.0 synthesis did not affect the expression of collagen I as well as procontractile peptides such as alpha-smooth muscle actin, and transforming growth factor-beta1 had no effect on FGFR1OP2/wit3.0 expression. Fibroblastic cells derived from embryonic stem cells carrying FGFR1OP2/wit3.0 (+/-) mutation showed significant retardation in cell migration. Thus, we postulate that FGFR1OP2/wit3.0 may regulate cell motility and stimulate wound closure. FGFR1OP2/wit3.0 was not up-regulated during skin wound healing; however, when treated with FGFR1OP2/wit3.0 -expression vector, the skin wound closure was significantly accelerated, resulting in the limited granulation tissue formation. Our data suggest that FGFR1OP2/wit3.0 may possess a therapeutic potential for wound management.
Figures

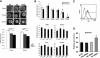


Similar articles
-
Oral fibroblast expression of wound-inducible transcript 3.0 (wit3.0) accelerates the collagen gel contraction in vitro.J Biol Chem. 2003 Dec 19;278(51):51527-34. doi: 10.1074/jbc.M309616200. Epub 2003 Oct 3. J Biol Chem. 2003. PMID: 14527947
-
High throughput screening of biologically functional small molecules for modulating the expression of FGFR1OP2/wit3.0 in fibroblasts.J Calif Dent Assoc. 2012 Dec;40(12):929-31, 934-7. J Calif Dent Assoc. 2012. PMID: 23362665
-
Wound closure and wound management: A new therapeutic molecular target.Cell Adh Migr. 2010 Jul-Sep;4(3):396-9. doi: 10.4161/cam.4.3.11917. Epub 2010 Jul 31. Cell Adh Migr. 2010. PMID: 20448469 Free PMC article.
-
A genetic association study of single nucleotide polymorphisms in FGFR1OP2/wit3.0 and long-term atrophy of edentulous mandible.PLoS One. 2011 Jan 19;6(1):e16204. doi: 10.1371/journal.pone.0016204. PLoS One. 2011. PMID: 21283824 Free PMC article.
-
Forceful closure: cytoskeletal networks in embryonic wound repair.Mol Biol Cell. 2019 Jun 1;30(12):1353-1358. doi: 10.1091/mbc.E18-04-0248. Mol Biol Cell. 2019. PMID: 31145669 Free PMC article. Review.
Cited by
-
FGFR1OP2-FGFR1 induced myeloid leukemia and T-cell lymphoma in a mouse model.Haematologica. 2016 Mar;101(3):e91-4. doi: 10.3324/haematol.2015.137695. Epub 2015 Nov 20. Haematologica. 2016. PMID: 26589915 Free PMC article. No abstract available.
-
Neuronal Wiskott-Aldrich syndrome protein (N-WASP) is critical for formation of α-smooth muscle actin filaments during myofibroblast differentiation.Am J Physiol Lung Cell Mol Physiol. 2012 Oct 15;303(8):L692-702. doi: 10.1152/ajplung.00390.2011. Epub 2012 Aug 10. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22886502 Free PMC article.
-
Biochemical and Biophysical Cues in Matrix Design for Chronic and Diabetic Wound Treatment.Tissue Eng Part B Rev. 2017 Feb;23(1):9-26. doi: 10.1089/ten.TEB.2016.0200. Epub 2016 Aug 19. Tissue Eng Part B Rev. 2017. PMID: 27405960 Free PMC article.
-
Proteome changes in platelets activated by arachidonic acid, collagen, and thrombin.Proteome Sci. 2010 Nov 12;8:56. doi: 10.1186/1477-5956-8-56. Proteome Sci. 2010. PMID: 21073729 Free PMC article.
-
Association between FGFR1OP2/wit3.0 polymorphisms and residual ridge resorption of mandible in Korean population.PLoS One. 2012;7(8):e42734. doi: 10.1371/journal.pone.0042734. Epub 2012 Aug 6. PLoS One. 2012. PMID: 22880093 Free PMC article.
References
-
- Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Singer AJ, Clark RAF. Cutaneous wound healing. New Eng J Med. 1999;341:738–746. - PubMed
-
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. - PubMed
-
- Darenfed H, Mandato CA. Wound-induced contractile ring: a model for cytokinesis. Biochem Cell Biol. 2005;83:711–720. - PubMed
-
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

