Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells
- PMID: 19959817
- PMCID: PMC2822503
- DOI: 10.1152/ajpgi.00409.2009
Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells
Abstract
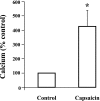
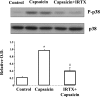
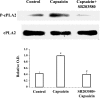
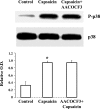
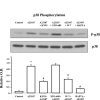
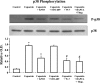
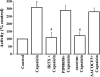
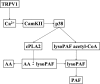
Transient receptor potential channel, vanilloid subfamily member 1 (TRPV1) receptors were identified in human esophageal squamous epithelial cell line HET-1A by RT-PCR and by Western blot. In fura-2 AM-loaded cells, the TRPV1 agonist capsaicin caused a fourfold cytosolic calcium increase, supporting a role of TRPV1 as a capsaicin-activated cation channel. Capsaicin increased production of platelet activating factor (PAF), an important inflammatory mediator that acts as a chemoattractant and activator of immune cells. The increase was reduced by the p38 MAP kinase (p38) inhibitor SB203580, by the cytosolic phospholipase A2 (cPLA(2)) inhibitor AACOCF3, and by the lyso-PAF acetyltransferase inhibitor sanguinarin, indicating that capsaicin-induced PAF production may be mediated by activation of cPLA(2), p38, and lyso-PAF acetyltransferase. To establish a sequential signaling pathway, we examined the phosphorylation of p38 and cPLA(2) by Western blot. Capsaicin induced phosphorylation of p38 and cPLA(2). Capsaicin-induced p38 phosphorylation was not affected by AACOCF3. Conversely, capsaicin-induced cPLA(2) phosphorylation was blocked by SB203580, indicating that capsaicin-induced PAF production depends on sequential activation of p38 and cPLA(2). To investigate how p38 phosphorylation may result from TRPV1-mediated calcium influx, we examined a possible role of calmodulin kinase (CaM-K). p38 phosphorylation was stimulated by the calcium ionophore A23187 and by capsaicin, and the response to both agonists was reduced by a CaM inhibitor and by CaM-KII inhibitors, indicating that calcium induced activation of CaM and CaM-KII results in P38 phosphorylation. Acetyl-CoA transferase activity increased in response to capsaicin and was inhibited by SB203580, indicating that p38 phosphorylation in turn causes activation of acetyl-CoA transferase to produce PAF. Thus epithelial cells produce PAF in response to TRPV1-mediated calcium elevation.
Figures


Similar articles
-
HCl-induced and ATP-dependent upregulation of TRPV1 receptor expression and cytokine production by human esophageal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G635-45. doi: 10.1152/ajpgi.00097.2012. Epub 2012 Jul 12. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22790593 Free PMC article.
-
ATP: a mediator for HCl-induced TRPV1 activation in esophageal mucosa.Am J Physiol Gastrointest Liver Physiol. 2011 Dec;301(6):G1075-82. doi: 10.1152/ajpgi.00336.2011. Epub 2011 Sep 29. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21960521 Free PMC article.
-
TRPV1-dependent ERK1/2 activation in porcine lens epithelium.Exp Eye Res. 2018 Jul;172:128-136. doi: 10.1016/j.exer.2018.04.006. Epub 2018 Apr 11. Exp Eye Res. 2018. PMID: 29654770 Free PMC article.
-
Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin.Curr Neurovasc Res. 2017;14(2):184-189. doi: 10.2174/1567202614666170313105337. Curr Neurovasc Res. 2017. PMID: 28294062 Free PMC article. Review.
-
TRPV1: Receptor structure, activation, modulation and role in neuro-immune interactions and pain.Cell Calcium. 2024 May;119:102870. doi: 10.1016/j.ceca.2024.102870. Epub 2024 Mar 8. Cell Calcium. 2024. PMID: 38531262 Review.
Cited by
-
HCl-induced and ATP-dependent upregulation of TRPV1 receptor expression and cytokine production by human esophageal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G635-45. doi: 10.1152/ajpgi.00097.2012. Epub 2012 Jul 12. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22790593 Free PMC article.
-
GPR84 and TREM-1 Signaling Contribute to the Pathogenesis of Reflux Esophagitis.Mol Med. 2016 Aug;21(1):1011-1024. doi: 10.2119/molmed.2015.00098. Epub 2016 May 9. Mol Med. 2016. PMID: 26650186 Free PMC article.
-
Involvement of the TRPV1 channel in the modulation of spontaneous locomotor activity, physical performance and physical exercise-induced physiological responses.Braz J Med Biol Res. 2016;49(6):e5183. doi: 10.1590/1414-431X20165183. Epub 2016 May 10. Braz J Med Biol Res. 2016. PMID: 27191606 Free PMC article. Review.
-
Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands.Am J Physiol Gastrointest Liver Physiol. 2015 Jun 1;308(11):G904-23. doi: 10.1152/ajpgi.00333.2014. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25882613 Free PMC article.
-
Upregulation of Vanilloid Receptor-1 in Functional Dyspepsia With or Without Helicobacter pylori Infection.Medicine (Baltimore). 2016 May;95(19):e3410. doi: 10.1097/MD.0000000000003410. Medicine (Baltimore). 2016. PMID: 27175641 Free PMC article.
References
-
- Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch Dermatol Res 292: 256–259, 2000 - PubMed
-
- Allen BG, Walsh MP. The biochemical basis of the regulation of smooth muscular contraction. Trends Biochem Sci 19: 362–368, 1994 - PubMed
-
- Baker PR, Owen JS, Nixon AB, Thomas LN, Wooten R, Daniel LW, O'Flaherty JT, Wykle RL. Regulation of platelet-activating factor synthesis in human neutrophils by MAP kinases. Biochim Biophys Acta 1592: 175–184, 2002 - PubMed
-
- Benveniste J, Chignard M. A role for PAF-acether (platelet-activating factor) in platelet-dependent vascular diseases? Circulation 72: 713–717, 1985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

