Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat
- PMID: 19959819
- PMCID: PMC2822499
- DOI: 10.1152/ajpgi.00396.2009
Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat
Abstract
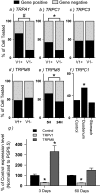
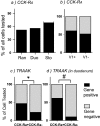
Vagal afferent neurons relay important information regarding the control of the gastrointestinal system. However, the ionic mechanisms that underlie vagal activation induced by sensory inputs are not completely understood. We postulate that transient receptor potential (TRP) channels and/or two-pore potassium (K2p) channels are targets for activating vagal afferents. In this study we explored the distribution of these channels in vagal afferents by quantitative PCR after a capsaicin treatment to eliminate capsaicin-sensitive neurons, and by single-cell PCR measurements in vagal afferent neurons cultured after retrograde labeling from the stomach or duodenum. We found that TRPC1/3/5/6, TRPV1-4, TRPM8, TRPA1, TWIK2, TRAAK, TREK1, and TASK1/2 were all present in rat nodose ganglia. Both lesion results and single-cell PCR results suggested that TRPA1 and TRPC1 were preferentially expressed in neurons that were either capsaicin sensitive or TRPV1 positive. Expression of TRPM8 varied dynamically after various manipulations, which perhaps explains the disparate results obtained by different investigators. Last, we also examined ion channel distribution with the A-type CCK receptor (CCK-R(A)) and found there was a significant preference for neurons that express TRAAK to also express CCK-R(A), especially in gut-innervating neurons. These findings, combined with findings from prior studies, demonstrated that background conductances such as TRPC1, TRPA1, and TRAAK are indeed differentially distributed in the nodose ganglia, and not only do they segregate with specific markers, but the degree of overlap is also dependent on the innervation target.
Figures



Similar articles
-
TRPM8 function and expression in vagal sensory neurons and afferent nerves innervating guinea pig esophagus.Am J Physiol Gastrointest Liver Physiol. 2015 Mar 15;308(6):G489-96. doi: 10.1152/ajpgi.00336.2014. Epub 2015 Jan 15. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25591866 Free PMC article.
-
Differential immunostaining patterns of transient receptor potential (TRP) ion channels in the rat nodose ganglion.J Anat. 2022 Aug;241(2):230-244. doi: 10.1111/joa.13656. Epub 2022 Apr 9. J Anat. 2022. PMID: 35396708 Free PMC article.
-
Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons.PLoS One. 2013 Oct 21;8(10):e77998. doi: 10.1371/journal.pone.0077998. eCollection 2013. PLoS One. 2013. PMID: 24205061 Free PMC article.
-
Interaction between TRPA1 and TRPV1: Synergy on pulmonary sensory nerves.Pulm Pharmacol Ther. 2015 Dec;35:87-93. doi: 10.1016/j.pupt.2015.08.003. Epub 2015 Aug 14. Pulm Pharmacol Ther. 2015. PMID: 26283426 Free PMC article. Review.
-
TRP channels in thermosensation.Curr Opin Neurobiol. 2022 Aug;75:102591. doi: 10.1016/j.conb.2022.102591. Epub 2022 Jun 18. Curr Opin Neurobiol. 2022. PMID: 35728275 Review.
Cited by
-
TRP channels in the digestive system.Curr Pharm Biotechnol. 2011 Jan 1;12(1):24-34. doi: 10.2174/138920111793937862. Curr Pharm Biotechnol. 2011. PMID: 20932260 Free PMC article. Review.
-
Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system.Pharmacol Ther. 2011 Jul;131(1):142-70. doi: 10.1016/j.pharmthera.2011.03.006. Epub 2011 Mar 21. Pharmacol Ther. 2011. PMID: 21420431 Free PMC article. Review.
-
TRPM8 function and expression in vagal sensory neurons and afferent nerves innervating guinea pig esophagus.Am J Physiol Gastrointest Liver Physiol. 2015 Mar 15;308(6):G489-96. doi: 10.1152/ajpgi.00336.2014. Epub 2015 Jan 15. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25591866 Free PMC article.
-
Cannabidiol activation of vagal afferent neurons requires TRPA1.J Neurophysiol. 2020 Nov 1;124(5):1388-1398. doi: 10.1152/jn.00128.2020. Epub 2020 Sep 23. J Neurophysiol. 2020. PMID: 32965166 Free PMC article.
-
Effects of acid on vagal nociceptive afferent subtypes in guinea pig esophagus.Am J Physiol Gastrointest Liver Physiol. 2014 Aug 15;307(4):G471-8. doi: 10.1152/ajpgi.00156.2014. Epub 2014 Jul 3. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24994852 Free PMC article.
References
-
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 278: 30429–30434, 2003 - PubMed
-
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci 20: 2276–2282, 2004 - PubMed
-
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000 - PubMed
-
- Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res 746: 195–206, 1997 - PubMed
-
- Bossu JL, Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci Lett 51: 241–246, 1984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

