Enforcing temporal control of maternal mRNA translation during oocyte cell-cycle progression
- PMID: 19959990
- PMCID: PMC2824450
- DOI: 10.1038/emboj.2009.337
Enforcing temporal control of maternal mRNA translation during oocyte cell-cycle progression
Abstract
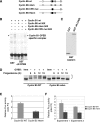
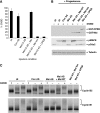
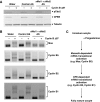
Meiotic cell-cycle progression in progesterone-stimulated Xenopus oocytes requires that the translation of pre-existing maternal mRNAs occur in a strict temporal order. Timing of translation is regulated through elements within the mRNA 3' untranslated region (3' UTR), which respond to cell cycle-dependant signalling. One element that has been previously implicated in the temporal control of mRNA translation is the cytoplasmic polyadenylation element (CPE). In this study, we show that the CPE does not direct early mRNA translation. Rather, early translation is directed through specific early factors, including the Musashi-binding element (MBE) and the MBE-binding protein, Musashi. Our findings indicate that although the cyclin B5 3' UTR contains both CPEs and an MBE, the MBE is the critical regulator of early translation. The cyclin B2 3' UTR contains CPEs, but lacks an MBE and is translationally activated late in maturation. Finally, utilizing antisense oligonucleotides to attenuate endogenous Musashi synthesis, we show that Musashi is critical for the initiation of early class mRNA translation and for the subsequent activation of CPE-dependant mRNA translation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Autoregulation of Musashi1 mRNA translation during Xenopus oocyte maturation.Mol Reprod Dev. 2012 Aug;79(8):553-63. doi: 10.1002/mrd.22060. Epub 2012 Jul 9. Mol Reprod Dev. 2012. PMID: 22730340 Free PMC article.
-
Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation.EMBO J. 2006 Jun 21;25(12):2792-801. doi: 10.1038/sj.emboj.7601159. Epub 2006 Jun 8. EMBO J. 2006. PMID: 16763568 Free PMC article.
-
Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes.J Biol Chem. 2004 Apr 23;279(17):17650-9. doi: 10.1074/jbc.M313837200. Epub 2004 Jan 29. J Biol Chem. 2004. PMID: 14752101 Free PMC article.
-
Translational control by cytoplasmic polyadenylation in Xenopus oocytes.Biochim Biophys Acta. 2008 Apr;1779(4):217-29. doi: 10.1016/j.bbagrm.2008.02.002. Epub 2008 Feb 14. Biochim Biophys Acta. 2008. PMID: 18316045 Free PMC article. Review.
-
Cytoplasmic mRNA polyadenylation and translation assays.Methods Mol Biol. 2006;322:183-98. doi: 10.1007/978-1-59745-000-3_13. Methods Mol Biol. 2006. PMID: 16739724 Review.
Cited by
-
Xenopus laevis zygote arrest 2 (zar2) encodes a zinc finger RNA-binding protein that binds to the translational control sequence in the maternal Wee1 mRNA and regulates translation.Dev Biol. 2012 Sep 15;369(2):177-90. doi: 10.1016/j.ydbio.2012.06.012. Epub 2012 Jun 23. Dev Biol. 2012. PMID: 22732570 Free PMC article.
-
Dueling RNA-binding proteins promote translational activation.Nat Struct Mol Biol. 2017 Aug 3;24(8):609-610. doi: 10.1038/nsmb.3445. Nat Struct Mol Biol. 2017. PMID: 28771463 No abstract available.
-
Musashi-dependent mRNA translational activation is mediated through association with the Scd6/Like-sm family member, LSM14B.Sci Rep. 2025 Apr 10;15(1):12363. doi: 10.1038/s41598-025-97188-9. Sci Rep. 2025. PMID: 40211036 Free PMC article.
-
Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition.Genes Dev. 2011 Apr 1;25(7):755-66. doi: 10.1101/gad.2028911. Genes Dev. 2011. PMID: 21460039 Free PMC article.
-
Musashi Exerts Control of Gonadotrope Target mRNA Translation During the Mouse Estrous Cycle.Endocrinology. 2023 Aug 1;164(9):bqad113. doi: 10.1210/endocr/bqad113. Endocrinology. 2023. PMID: 37477898 Free PMC article.
References
-
- Barkoff AF, Dickson KS, Gray NK, Wickens M (2000) Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation. Dev Biol 220: 97–109 - PubMed
-
- Castro A, Mandart E, Lorca T, Galas S (2003) Involvement of Aurora A kinase during meiosis I–II transition in Xenopus oocytes. J Biol Chem 278: 2236–2241 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

