Restoration of cerebral vascular relaxation in renin congenic rats by introgression of the Dahl R renin gene
- PMID: 19959997
- PMCID: PMC2924749
- DOI: 10.1038/ajh.2009.236
Restoration of cerebral vascular relaxation in renin congenic rats by introgression of the Dahl R renin gene
Abstract
Background: This study determined whether transfer of the renin gene from the Dahl salt-resistant (Dahl R) strain into the Dahl salt-sensitive (SS) genetic background restores the relaxation of middle cerebral arteries (MCAs) to different vasodilator stimuli in S/renRR renin congenic (SS.SR-(D13N1 and Syt2)/Mcwi) (RGRR) rats maintained on low-salt (0.4% NaCl) diet.
Methods: Responses to vasodilator stimuli were evaluated in isolated MCA from SS (Dahl SS/Jr/Hsd/MCWi), RGRR rats, and Dahl R rats.
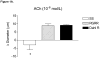
Results: MCA from SS rats failed to dilate in response to acetylcholine (ACh; 10(-6) mol/l), hypoxia (PO2 reduction to 40-45 mm Hg), and iloprost (10(-11) g/ml). ACh- and hypoxia-induced dilations were present in Dahl R rats and restored in RGRR rats. MCA from RGRR and SS constricted in response to iloprost, whereas MCA from Dahl R rats dilated in response to iloprost. MCA from SS, RGRR, and Dahl R rats exhibited similar dilations in response to cholera toxin (10(-9) g/ml) and dialated in response to the nitric oxide (NO) donor DEA-NONOate (10(-5) mol/l).
Conclusions: (i) Restoration of normal regulation of the renin-angiotensin system restores dilations to ACh and hypoxia that are impaired in SS rats, (ii) prostacyclin signaling is impaired in SS and RGRR rats but intact in Dahl R rats, indicating that alleles other than the renin gene affect vascular relaxation in response to this agonist; and (iii) vascular smooth muscle sensitivity to NO is preserved in SS and RGRR and is not responsible for impaired arterial relaxation in response to ACh in SS rats.
Conflict of interest statement
Figures







References
-
- Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32. - PubMed
-
- Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39:41–50. - PubMed
-
- Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14. - PubMed
-
- Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2003;284:H1124–H1133. - PubMed
-
- Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H2196–H2202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

