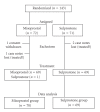
A randomized controlled trial of misoprostol and sulprostone to end pregnancy after fetal death
- PMID: 19960062
- PMCID: PMC2778817
- DOI: 10.1155/2009/496320
A randomized controlled trial of misoprostol and sulprostone to end pregnancy after fetal death
Abstract
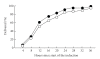
Objective. To compare effectiveness, side effects, and patients' perception of vaginal misoprostol versus intravenous sulprostone for ending pregnancy after fetal death between 14 and 42 weeks gestation. Method. Multicenter randomized controlled trial, using block randomization, central allocation, and prior power analysis. Outcome measures. Induction-delivery interval, gastrointestinal side effects, use of analgesia, pain perception, pyrexia, placental retention, hemorrhage, and women's opinions. Results. Of 176 women aimed for, 143 were randomized over 7 years, of whom 4 were excluded. There was no difference in delivery within 24 and 36 hours: 91.4% and 97.1% with misoprostol (n = 70) versus 85.5% and 92.8% with sulprostone (n = 69). There was no difference in either gastrointestinal side effects, as reported by the women and their caregivers, use of analgesia, women's pain perception, blood loss or placental retention. Hyperthermia >/=38 degrees C was more common with misoprostol (24.3%) than with sulprostone (11.6%; difference: +12.7%; 95% CI: +1.2% to +25.3%) and related to the total dose used. Acceptability of both induction methods was similar except for freedom of movement, which was substantially in favor of misoprostol (lack of freedom reported with misoprostol in 34.3% versus 63.8% with sulprostone; difference: -29.5%; 95% CI: -13.6% to -45.4%). Conclusions. Misoprostol and sulprostone are similarly effective with little difference in side effects except for hyperthermia, related to the dose of misoprostol used, and women's reported lack of mobility with intravenous sulprostone. Effectiveness of both methods increased with gestational age.
Figures
Similar articles
-
[Randomized study of sulprostone versus misoprostol in the cervical preparation before elective abortion in nulliparous women].J Gynecol Obstet Biol Reprod (Paris). 1995;24(5):505-10. J Gynecol Obstet Biol Reprod (Paris). 1995. PMID: 7499737 Clinical Trial. French.
-
[Termination of pregnancy in the 2nd trimester: mifepriston/misoprostol preferable to sulprostone].Ned Tijdschr Geneeskd. 2009;153:A138. Ned Tijdschr Geneeskd. 2009. PMID: 19930731 Dutch.
-
Medical management for termination of second and third trimester pregnancies: a comparison of strategies.Eur J Obstet Gynecol Reprod Biol. 2004 Sep 10;116(1):16-21. doi: 10.1016/j.ejogrb.2003.12.012. Eur J Obstet Gynecol Reprod Biol. 2004. PMID: 15294361
-
Misoprostol for induction of labour at term: a more effective agent than dinoprostone vaginal gel.Br J Obstet Gynaecol. 1999 Aug;106(8):793-7. doi: 10.1111/j.1471-0528.1999.tb08399.x. Br J Obstet Gynaecol. 1999. PMID: 10453828 Clinical Trial.
-
Comparison of vaginal and sublingual misoprostol for second trimester abortion: randomized controlled equivalence trial.Hum Reprod. 2009 Jan;24(1):106-12. doi: 10.1093/humrep/den328. Epub 2008 Sep 14. Hum Reprod. 2009. PMID: 18794161 Clinical Trial.
Cited by
-
Route of Delivery in Women With Stillbirth: Results From the Stillbirth Collaborative Research Network.Obstet Gynecol. 2017 Apr;129(4):693-698. doi: 10.1097/AOG.0000000000001935. Obstet Gynecol. 2017. PMID: 28333794 Free PMC article.
-
Medical treatment for early fetal death (less than 24 weeks).Cochrane Database Syst Rev. 2019 Jun 17;6(6):CD002253. doi: 10.1002/14651858.CD002253.pub4. Cochrane Database Syst Rev. 2019. PMID: 31206170 Free PMC article.
-
Methods for managing miscarriage: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jun 1;6(6):CD012602. doi: 10.1002/14651858.CD012602.pub2. Cochrane Database Syst Rev. 2021. PMID: 34061352 Free PMC article.
-
Medical treatment for early fetal death (less than 24 weeks).Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD002253. doi: 10.1002/14651858.CD002253.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 Jun 17;6:CD002253. doi: 10.1002/14651858.CD002253.pub4. PMID: 16855990 Free PMC article. Updated.
References
-
- Pritchard JA. Fetal death in utero. Obstetrics & Gynecology. 1959;14:573–580. - PubMed
-
- Grandin DJ, Hall RE. Fetal death before the onset of labor: an analysis of 407 cases. American Journal of Obstetrics and Gynecology. 1960;79(2):237–243. - PubMed
-
- Anderson ABM, Turnbull AC. Spontaneous contractility and oxytocin sensitivity of the human uterus in mid-pregnancy. The Journal of Obstetrics and Gynaecology of the British Commonwealth. 1968;75(3):271–277. - PubMed
-
- Anderson ABM, Turnbull AC. Relationship between length of gestation and cervical dilatation, uterine contractility, and other factors during pregnancy. American Journal of Obstetrics and Gynecology. 1969;105(8):1207–1214. - PubMed
-
- Keirse MJNC. Termination of pregnancy after intrauterine foetal death. In: Keirse MJNC, Bennebroek Gravenhorst J, Van Lith DAF, Embrey MP, editors. Second Trimester Pregnancy Termination. The Hague, The Netherlands: Leiden University Press; 1982. pp. 138–154.
LinkOut - more resources
Full Text Sources