Is Mn-Bound Substrate Water Protonated in the S(2) State of Photosystem II?
- PMID: 19960065
- PMCID: PMC2784071
- DOI: 10.1007/s00723-009-0051-1
Is Mn-Bound Substrate Water Protonated in the S(2) State of Photosystem II?
Abstract
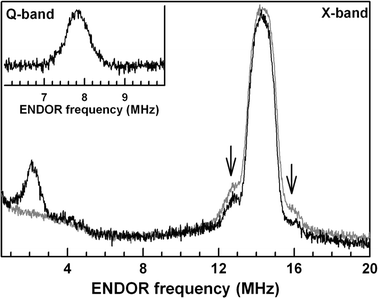
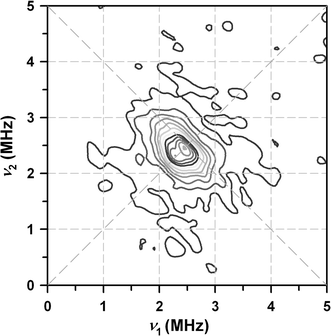
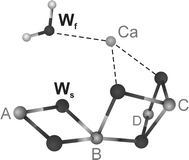
In spite of great progress in resolving the geometric structure of the water-splitting Mn(4)O(x)Ca cluster in photosystem II, the binding sites and modes of the two substrate water molecules are still insufficiently characterized. While time-resolved membrane-inlet mass spectrometry measurements indicate that both substrate water molecules are bound to the oxygen-evolving complex (OEC) in the S(2) and S(3) states (Hendry and Wydrzynski in Biochemistry 41:13328-13334, 2002), it is not known (1) if they are both Mn-bound, (2) if they are terminal or bridging ligands, and (3) in what protonation state they are bound in the different oxidation states S(i) (i = 0, 1, 2, 3, 4) of the OEC. By employing (17)O hyperfine sublevel correlation (HYSCORE) spectroscopy we recently demonstrated that in the S(2) state there is only one (type of) Mn-bound oxygen that is water exchangeable. We therefore tentatively identified this oxygen as one substrate 'water' molecule, and on the basis of the finding that it has a hyperfine interaction of about 10 MHz with the electron spin of the Mn(4)O(x)Ca cluster, we suggest that it is bound as a Mn-O-Mn bridge within a bis-mu(2) oxo-bridged unit (Su et al. in J Am Chem Soc 130:786-787, 2008). Employing pulse electron paramagnetic resonance, (1)H/(2)H Mims electron-nuclear double resonance and (2)H-HYSCORE spectroscopies together with (1)H/(2)H-exchange here, we test this hypothesis by probing the protonation state of this exchangeable oxygen. We conclude that this oxygen is fully deprotonated. This result is discussed in the light of earlier reports in the literature.
Figures


Similar articles
-
EPR-ENDOR characterization of (17O, 1H, 2H) water in manganese catalase and its relevance to the oxygen-evolving complex of photosystem II.J Am Chem Soc. 2012 Jan 25;134(3):1504-12. doi: 10.1021/ja203465y. Epub 2012 Jan 9. J Am Chem Soc. 2012. PMID: 22142421 Free PMC article.
-
Detection of the water-binding sites of the oxygen-evolving complex of Photosystem II using W-band 17O electron-electron double resonance-detected NMR spectroscopy.J Am Chem Soc. 2012 Oct 10;134(40):16619-34. doi: 10.1021/ja3053267. Epub 2012 Sep 27. J Am Chem Soc. 2012. PMID: 22937979
-
Mono-manganese mechanism of the photosystem II water splitting reaction by a unique Mn4Ca cluster.Biochim Biophys Acta. 2007 Jun;1767(6):484-92. doi: 10.1016/j.bbabio.2007.03.012. Epub 2007 Apr 4. Biochim Biophys Acta. 2007. PMID: 17490604 Review.
-
Probing mode and site of substrate water binding to the oxygen-evolving complex in the S2 state of photosystem II by 17O-HYSCORE spectroscopy.J Am Chem Soc. 2008 Jan 23;130(3):786-7. doi: 10.1021/ja076620i. J Am Chem Soc. 2008. PMID: 18161970
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
Cited by
-
Participation of glutamate-354 of the CP43 polypeptide in the ligation of manganese and the binding of substrate water in photosystem II.Biochemistry. 2011 Jan 11;50(1):63-81. doi: 10.1021/bi1015937. Epub 2010 Dec 8. Biochemistry. 2011. PMID: 21114287 Free PMC article.
-
Calcium in the oxygen-evolving complex: structural and mechanistic role determined by X-ray spectroscopy.J Photochem Photobiol B. 2011 Jul-Aug;104(1-2):51-9. doi: 10.1016/j.jphotobiol.2011.02.019. Epub 2011 Mar 3. J Photochem Photobiol B. 2011. PMID: 21524917 Free PMC article. Review.
-
EPR-ENDOR characterization of (17O, 1H, 2H) water in manganese catalase and its relevance to the oxygen-evolving complex of photosystem II.J Am Chem Soc. 2012 Jan 25;134(3):1504-12. doi: 10.1021/ja203465y. Epub 2012 Jan 9. J Am Chem Soc. 2012. PMID: 22142421 Free PMC article.
References
-
- Lubitz W, Reijerse EJ, Messinger J. Energy Environ. Sci. 2008;1:15–31. doi: 10.1039/b808792j. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
