Nicotinamide overload may play a role in the development of type 2 diabetes
- PMID: 19960564
- PMCID: PMC2789220
- DOI: 10.3748/wjg.15.5674
Nicotinamide overload may play a role in the development of type 2 diabetes
Abstract
Aim: To investigate whether nicotinamide overload plays a role in type 2 diabetes.
Methods: Nicotinamide metabolic patterns of 14 diabetic and 14 non-diabetic subjects were compared using HPLC. Cumulative effects of nicotinamide and N(1)-methylnicotinamide on glucose metabolism, plasma H(2)O(2) levels and tissue nicotinamide adenine dinucleotide (NAD) contents of adult Sprague-Dawley rats were observed. The role of human sweat glands and rat skin in nicotinamide metabolism was investigated using sauna and burn injury, respectively.
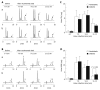
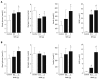
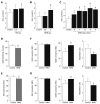
Results: Diabetic subjects had significantly higher plasma N(1)-methylnicotinamide levels 5 h after a 100-mg nicotinamide load than the non-diabetic subjects (0.89 +/- 0.13 micromol/L vs 0.6 +/- 0.13 micromol/L, P < 0.001). Cumulative doses of nicotinamide (2 g/kg) significantly increased rat plasma N(1)-methylnicotinamide concentrations associated with severe insulin resistance, which was mimicked by N(1)-methylnicotinamide. Moreover, cumulative exposure to N(1)-methylnicotinamide (2 g/kg) markedly reduced rat muscle and liver NAD contents and erythrocyte NAD/NADH ratio, and increased plasma H(2)O(2) levels. Decrease in NAD/NADH ratio and increase in H(2)O(2) generation were also observed in human erythrocytes after exposure to N(1)-methylnicotinamide in vitro. Sweating eliminated excessive nicotinamide (5.3-fold increase in sweat nicotinamide concentration 1 h after a 100-mg nicotinamide load). Skin damage or aldehyde oxidase inhibition with tamoxifen or olanzapine, both being notorious for impairing glucose tolerance, delayed N(1)-methylnicotinamide clearance.
Conclusion: These findings suggest that nicotinamide overload, which induced an increase in plasma N(1)-methylnicotinamide, associated with oxidative stress and insulin resistance, plays a role in type 2 diabetes.
Figures




References
-
- Meigs JB, Larson MG, Fox CS, Keaney JF Jr, Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care. 2007;30:2529–2535. - PubMed
-
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 Suppl 2:S170–S180. - PubMed
-
- Andreassi MG. Metabolic syndrome, diabetes and atherosclerosis: influence of gene-environment interaction. Mutat Res. 2009;667:35–43. - PubMed
-
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. - PubMed
-
- Roche HM, Phillips C, Gibney MJ. The metabolic syndrome: the crossroads of diet and genetics. Proc Nutr Soc. 2005;64:371–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

