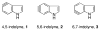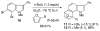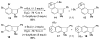Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions
- PMID: 19961152
- PMCID: PMC4175992
- DOI: 10.1021/ol902415s
Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions
Abstract
The 6,7-indolyne shows remarkable regioselectivity in its cycloaddition with 2-substituted furans. Electron-donating groups give predominantly the more sterically crowded product, while electron-withdrawing groups display the opposite regioselectivity. By contrast, 4,5- and 5,6-indolynes show no regioselectivity. Optimized electronic structure calculations using the M06-2X density functional and 6-311+G(2df,p) basis set revealed that the 6,7-indolynes are highly polar structures and that their cycloadditions have substantial electrophilic substitution character that leads to the observed preference for contrasteric products.
Figures
Similar articles
-
Indolyne and aryne distortions and nucleophilic regioselectivites.J Am Chem Soc. 2010 Feb 3;132(4):1267-9. doi: 10.1021/ja9098643. J Am Chem Soc. 2010. PMID: 20058924 Free PMC article.
-
Concise total synthesis of (+/-)-cis-trikentrin A and (+/-)-herbindole A via intermolecular indole aryne cycloaddition.Org Lett. 2009 Jan 1;11(1):201-4. doi: 10.1021/ol802425m. Org Lett. 2009. PMID: 19055375 Free PMC article.
-
Regioselective Diels-Alder cycloadditions and other reactions of 4,5-, 5,6-, and 6,7-indole arynes.Tetrahedron Lett. 2009 Jan 7;50(1):63-65. doi: 10.1016/j.tetlet.2008.10.086. Tetrahedron Lett. 2009. PMID: 21057588 Free PMC article.
-
Indolyne experimental and computational studies: synthetic applications and origins of selectivities of nucleophilic additions.J Am Chem Soc. 2010 Dec 22;132(50):17933-44. doi: 10.1021/ja1086485. Epub 2010 Nov 29. J Am Chem Soc. 2010. PMID: 21114321 Free PMC article.
-
Reactivity of 3-nitroindoles with electron-rich species.Chem Commun (Camb). 2021 Jan 5;57(1):27-44. doi: 10.1039/d0cc06658c. Chem Commun (Camb). 2021. PMID: 33300929 Review.
Cited by
-
Arylhydrazine Trapping of Benzynes: Mechanistic Insights and a Route to Azoarenes.Org Lett. 2021 May 7;23(9):3432-3436. doi: 10.1021/acs.orglett.1c00897. Epub 2021 Apr 19. Org Lett. 2021. PMID: 33872032 Free PMC article.
-
Expanding the Strained Alkyne Toolbox: Generation and Utility of Oxygen-Containing Strained Alkynes.J Am Chem Soc. 2016 Apr 13;138(14):4948-54. doi: 10.1021/jacs.6b01986. Epub 2016 Mar 29. J Am Chem Soc. 2016. PMID: 26987257 Free PMC article.
-
CuI -Mediated Bromoalkynylation and Hydroalkynylation Reactions of Unsymmetrical Benzynes: Complementary Modes of Addition.Angew Chem Int Ed Engl. 2018 Dec 10;57(50):16564-16568. doi: 10.1002/anie.201811783. Epub 2018 Nov 15. Angew Chem Int Ed Engl. 2018. PMID: 30390400 Free PMC article.
-
Reactions of hexadehydro-Diels-Alder benzynes with structurally complex multifunctional natural products.Nat Chem. 2017 Jun;9(6):523-530. doi: 10.1038/nchem.2732. Epub 2017 Feb 27. Nat Chem. 2017. PMID: 28537589 Free PMC article.
-
Mechanisms by which synthetic 6,7-annulated-4-substituted indole compounds with anti-proliferative activity disrupt mitosis and block cytokinesis in human HL-60 tumor cells in vitro.Anticancer Res. 2014 Apr;34(4):1643-55. Anticancer Res. 2014. PMID: 24692693 Free PMC article.
References
-
-
Synthetic studies, see: Smith AB, III, Kuerti L, Davulcu AH, Cho YS, Ohmoto K. J Org Chem. 2007;72:4611–4620.
-
-
- Dangel BD, Godula K, Youn SW, Sezen B, Sames D. J Am Chem Soc. 2002;124:11856–11857. - PubMed
- Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1987;38:3671–3674.
-
- Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org Lett. 2007;9:4135–4137. - PubMed
-
-
Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65.Another o-silyltriflate method for 4,5-indolyne formation and further cycloaddition reactions have subsequently been reported by Garg: Bronner SM, Bahnck KB, Garg NK. Org Lett. 2009;11:1007–1010.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources