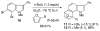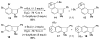Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions
- PMID: 19961152
- PMCID: PMC4175992
- DOI: 10.1021/ol902415s
Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions
Abstract
The 6,7-indolyne shows remarkable regioselectivity in its cycloaddition with 2-substituted furans. Electron-donating groups give predominantly the more sterically crowded product, while electron-withdrawing groups display the opposite regioselectivity. By contrast, 4,5- and 5,6-indolynes show no regioselectivity. Optimized electronic structure calculations using the M06-2X density functional and 6-311+G(2df,p) basis set revealed that the 6,7-indolynes are highly polar structures and that their cycloadditions have substantial electrophilic substitution character that leads to the observed preference for contrasteric products.
Figures
References
-
-
Synthetic studies, see: Smith AB, III, Kuerti L, Davulcu AH, Cho YS, Ohmoto K. J Org Chem. 2007;72:4611–4620.
-
-
- Dangel BD, Godula K, Youn SW, Sezen B, Sames D. J Am Chem Soc. 2002;124:11856–11857. - PubMed
- Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1987;38:3671–3674.
-
- Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org Lett. 2007;9:4135–4137. - PubMed
-
-
Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65.Another o-silyltriflate method for 4,5-indolyne formation and further cycloaddition reactions have subsequently been reported by Garg: Bronner SM, Bahnck KB, Garg NK. Org Lett. 2009;11:1007–1010.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources