Comparison of nine programs predicting pK(a) values of pharmaceutical substances
- PMID: 19961204
- PMCID: PMC7289148
- DOI: 10.1021/ci900289x
Comparison of nine programs predicting pK(a) values of pharmaceutical substances
Abstract
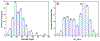
Knowledge of the possible ionization states of a pharmaceutical substance, embodied in the pK(a) values (logarithm of the acid dissociation constant), is vital for understanding many properties essential to drug development. We compare nine commercially available or free programs for predicting ionization constants. Eight of these programs are based on empirical methods: ACD/pK(a) DB 12.0, ADME Boxes 4.9, ADMET Predictor 3.0, Epik 1.6, Marvin 5.1.4, Pallas pKalc Net 2.0, Pipeline Pilot 5.0, and SPARC 4.2; one program is based on a quantum chemical method: Jaguar 7.5. We compared their performances by applying them to 197 pharmaceutical substances with 261 carefully determined and highly reliable experimental pK(a) values from a literature source. The programs ADME Boxes 4.9, ACD/pK(a) DB 12.0, and SPARC 4.2 ranked as the top three with mean absolute deviations of 0.389, 0.478, and 0.651 and r(2) values of 0.944, 0.908, and 0.894, respectively. ACD/pK(a) DB 12.0 predicted all sites, whereas ADME Boxes 4.9 and SPARC 4.2 failed to predict 5 and 18 sites, respectively. The performance of the quantum chemical-based program Jaguar 7.5 was not as expected, with a mean absolute deviation of 1.283 and an r(2) value of 0.579, indicating the potential for further development of this type of approach to pK(a) prediction.
Figures





Similar articles
-
Evaluation of pKa estimation methods on 211 druglike compounds.J Chem Inf Model. 2010 Apr 26;50(4):565-71. doi: 10.1021/ci100019p. J Chem Inf Model. 2010. PMID: 20225863
-
Benchmarking and validating algorithms that estimate pK(a) values of drugs based on their molecular structures.Anal Bioanal Chem. 2007 Oct;389(4):1267-81. doi: 10.1007/s00216-007-1502-x. Epub 2007 Aug 4. Anal Bioanal Chem. 2007. PMID: 17676314
-
Epik: a software program for pK( a ) prediction and protonation state generation for drug-like molecules.J Comput Aided Mol Des. 2007 Dec;21(12):681-91. doi: 10.1007/s10822-007-9133-z. Epub 2007 Sep 27. J Comput Aided Mol Des. 2007. PMID: 17899391
-
High-throughput pKa screening and prediction amenable for ADME profiling.Expert Opin Drug Metab Toxicol. 2006 Feb;2(1):139-55. doi: 10.1517/17425255.2.1.139. Expert Opin Drug Metab Toxicol. 2006. PMID: 16863474 Review.
-
Another string to your bow: machine learning prediction of the pharmacokinetic properties of small molecules.Expert Opin Drug Discov. 2024 Jun;19(6):683-698. doi: 10.1080/17460441.2024.2348157. Epub 2024 May 10. Expert Opin Drug Discov. 2024. PMID: 38727016 Review.
Cited by
-
Bridging Machine Learning and Thermodynamics for Accurate pK a Prediction.JACS Au. 2024 Jul 17;4(9):3451-3465. doi: 10.1021/jacsau.4c00271. eCollection 2024 Sep 23. JACS Au. 2024. PMID: 39328749 Free PMC article.
-
Warfarin: history, tautomerism and activity.J Comput Aided Mol Des. 2010 Jun;24(6-7):553-73. doi: 10.1007/s10822-010-9335-7. Epub 2010 Mar 30. J Comput Aided Mol Des. 2010. PMID: 20352297 Review.
-
Dimorphite-DL: an open-source program for enumerating the ionization states of drug-like small molecules.J Cheminform. 2019 Feb 14;11(1):14. doi: 10.1186/s13321-019-0336-9. J Cheminform. 2019. PMID: 30767086 Free PMC article.
-
Novel ligands for a purine riboswitch discovered by RNA-ligand docking.Chem Biol. 2011 Mar 25;18(3):324-35. doi: 10.1016/j.chembiol.2010.12.020. Chem Biol. 2011. PMID: 21439477 Free PMC article.
-
Understanding the Exceptional Properties of Nitroacetamides in Water: A Computational Model Including the Solvent.Molecules. 2018 Dec 13;23(12):3308. doi: 10.3390/molecules23123308. Molecules. 2018. PMID: 30551625 Free PMC article.
References
-
- Comer J; Tam K In Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical, and Computational Strategies; Testa B, Waterbeemd H, Folkers G, Guy R, Eds.; Wiley-VCH: Weinheim; New York, 2001; pp 275–304.
-
- Comer JEA In Comprehensive Medicinal Chemistry II. Testa B, Waterbeemd H, Eds.; Elsevier: Oxford, UK, 2007; Vol. 5, pp 357–398.
-
- Mitra R; Shyam R; Mitra I; Miteva MA; Alexov E Calculating the protonation states of proteins and small molecules: Implications to ligand-receptor interactions. Curr. Comput-Aided Drug Des. 2008, 4, 169–179.
-
- Prue JE Ionic Equilibria. Pergamon Press: Oxford, New York, 1966.
-
- Poole SK; Patel S; Dehring K; Workman H; Poole CF Determination of acid dissociation constants by capillary electrophoresis. J. Chromatogr., A 2004, 1037, 445–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

