Photoswitching mechanism of cyanine dyes
- PMID: 19961226
- PMCID: PMC2797371
- DOI: 10.1021/ja904588g
Photoswitching mechanism of cyanine dyes
Abstract
Cyanine dyes have been shown to undergo reversible photoswitching, where the fluorophore can be switched between a fluorescent state and a dark state upon illumination at different wavelengths. The photochemical mechanism by which switching occurs has yet to be elucidated. In this study, we have determined the mechanism of photoswitching by characterizing the kinetics of dark state formation and the spectral and structural properties of the dark state. The rate of switching to the dark state depends on the concentration of the primary thiol in the solution and the solution pH in a manner quantitatively consistent with the formation of an encounter complex between the cyanine dye and ionized thiol prior to their conjugation. Mass spectrometry suggests that the photoconversion product is a thiol-cyanine adduct in which covalent attachment of the thiol to the polymethine bridge disrupts the original conjugated pi-electron system of the dye.
Figures

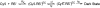

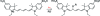
Similar articles
-
Unifying Mechanism for Thiol-Induced Photoswitching and Photostability of Cyanine Dyes.J Am Chem Soc. 2020 Jul 22;142(29):12681-12689. doi: 10.1021/jacs.0c03786. Epub 2020 Jul 13. J Am Chem Soc. 2020. PMID: 32594743 Free PMC article.
-
Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments.Q Rev Biophys. 2011 Feb;44(1):123-51. doi: 10.1017/S0033583510000247. Epub 2010 Nov 26. Q Rev Biophys. 2011. PMID: 21108866 Review.
-
Mechanism of Cyanine5 to Cyanine3 Photoconversion and Its Application for High-Density Single-Particle Tracking in a Living Cell.J Am Chem Soc. 2021 Sep 8;143(35):14125-14135. doi: 10.1021/jacs.1c04178. Epub 2021 Aug 25. J Am Chem Soc. 2021. PMID: 34432445
-
Tailoring cyanine dark states for improved optically modulated fluorescence recovery.J Phys Chem B. 2015 Apr 2;119(13):4637-43. doi: 10.1021/acs.jpcb.5b00777. Epub 2015 Mar 25. J Phys Chem B. 2015. PMID: 25763888 Free PMC article.
-
Productive Manipulation of Cyanine Dye π-Networks.Angew Chem Int Ed Engl. 2019 Jul 1;58(27):8974-8976. doi: 10.1002/anie.201902956. Epub 2019 May 24. Angew Chem Int Ed Engl. 2019. PMID: 31124257 Free PMC article. Review.
Cited by
-
Repurposing Cyanine Photoinstability To Develop Near-Infrared Light-Activatable Nanogels for In Vivo Cargo Delivery.J Am Chem Soc. 2022 Oct 5;144(39):18101-18108. doi: 10.1021/jacs.2c08187. Epub 2022 Sep 25. J Am Chem Soc. 2022. PMID: 36153991 Free PMC article.
-
Super-resolution Microscopy with Single Molecules in Biology and Beyond-Essentials, Current Trends, and Future Challenges.J Am Chem Soc. 2020 Oct 21;142(42):17828-17844. doi: 10.1021/jacs.0c08178. Epub 2020 Oct 9. J Am Chem Soc. 2020. PMID: 33034452 Free PMC article. Review.
-
From single molecules to life: microscopy at the nanoscale.Anal Bioanal Chem. 2016 Oct;408(25):6885-911. doi: 10.1007/s00216-016-9781-8. Epub 2016 Sep 9. Anal Bioanal Chem. 2016. PMID: 27613013 Free PMC article. Review.
-
Amorphous Quantum Nanomaterials.Adv Mater. 2019 Feb;31(5):e1806993. doi: 10.1002/adma.201806993. Epub 2018 Dec 5. Adv Mater. 2019. PMID: 30516861 Free PMC article.
-
Study of conformational transitions of i-motif DNA using time-resolved fluorescence and multivariate analysis methods.Nucleic Acids Res. 2019 Jul 26;47(13):6590-6605. doi: 10.1093/nar/gkz522. Nucleic Acids Res. 2019. PMID: 31199873 Free PMC article.
References
-
- Betzig E.; Patterson G. H.; Sougrat R.; Lindwasser O. W.; Olenych S.; Bonifacino J. S.; Davidson M. W.; Lippincott-Schwartz J.; Hess H. F. Science 2006, 313, 1642. - PubMed
-
- Fölling J.; Belov V.; Kunetsky R.; Medda R.; Schönle A.; Egner A.; Eggeling C.; Bossi M.; Hell S. W. Angew. Chem., Int. Ed. 2007, 46, 6266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

