Diversity, phylogenetic distribution, and origins of venomous catfishes
- PMID: 19961571
- PMCID: PMC2791775
- DOI: 10.1186/1471-2148-9-282
Diversity, phylogenetic distribution, and origins of venomous catfishes
Abstract
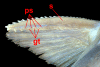
Background: The study of venomous fishes is in a state of relative infancy when compared to that of other groups of venomous organisms. Catfishes (Order Siluriformes) are a diverse group of bony fishes that have long been known to include venomous taxa, but the extent and phylogenetic distribution of this venomous species diversity has never been documented, while the nature of the venoms themselves also remains poorly understood. In this study, I used histological preparations from over 100 catfish genera, basic biochemical and toxicological analyses of fin spine extracts from several species, and previous systematic studies of catfishes to examine the distribution of venom glands in this group. These results also offer preliminary insights into the evolutionary history of venom glands in the Siluriformes.
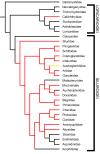
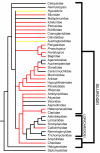
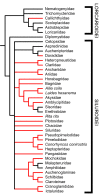
Results: Histological examinations of 158 catfish species indicate that approximately 1250-1625+ catfish species should be presumed to be venomous, when viewed in conjunction with several hypotheses of siluriform phylogeny. Maximum parsimony character optimization analyses indicate two to three independent derivations of venom glands within the Siluriformes. A number of putative toxic peptides were identified in the venoms of catfish species from many of the families determined to contain venomous representatives. These peptides elicit a wide array of physiological effects in other fishes, though any one species examined produced no more than three distinct putative toxins in its venom. The molecular weights and effects produced by these putative toxic peptides show strong similarities to previously characterized toxins found in catfish epidermal secretions.
Conclusion: Venom glands have evolved multiple times in catfishes (Order Siluriformes), and venomous catfishes may outnumber the combined diversity of all other venomous vertebrates. The toxic peptides found in catfish venoms may be derived from epidermal secretions that have been demonstrated to accelerate the healing of wounds, rather than defensive crinotoxins.
Figures




Similar articles
-
Venom evolution widespread in fishes: a phylogenetic road map for the bioprospecting of piscine venoms.J Hered. 2006 May-Jun;97(3):206-17. doi: 10.1093/jhered/esj034. Epub 2006 Jun 1. J Hered. 2006. PMID: 16740627
-
Evolution of Venomous Cartilaginous and Ray-Finned Fishes.Integr Comp Biol. 2016 Nov;56(5):950-961. doi: 10.1093/icb/icw070. Epub 2016 Jul 3. Integr Comp Biol. 2016. PMID: 27375272
-
A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences.Mol Phylogenet Evol. 2006 Dec;41(3):636-62. doi: 10.1016/j.ympev.2006.05.044. Epub 2006 Jun 10. Mol Phylogenet Evol. 2006. PMID: 16876440
-
Quo vadis venomics? A roadmap to neglected venomous invertebrates.Toxins (Basel). 2014 Dec 19;6(12):3488-551. doi: 10.3390/toxins6123488. Toxins (Basel). 2014. PMID: 25533518 Free PMC article. Review.
-
Advances in Reproductive Endocrinology and Neuroendocrine Research Using Catfish Models.Cells. 2021 Oct 20;10(11):2807. doi: 10.3390/cells10112807. Cells. 2021. PMID: 34831032 Free PMC article. Review.
Cited by
-
The complete mitogenome of Amazonian Brachyplatystoma filamentosum and the evolutionary history of body size in the order Siluriformes.Sci Rep. 2025 Mar 21;15(1):9873. doi: 10.1038/s41598-025-94272-y. Sci Rep. 2025. PMID: 40119108 Free PMC article.
-
Adding insult to injury: A review of infections following envenomings.Toxicon X. 2025 Jun 23;27:100230. doi: 10.1016/j.toxcx.2025.100230. eCollection 2025 Sep. Toxicon X. 2025. PMID: 40678361 Free PMC article. Review.
-
Competition and phylogeny determine community structure in Müllerian co-mimics.Nature. 2011 Jan 6;469(7328):84-8. doi: 10.1038/nature09660. Nature. 2011. PMID: 21209663
-
Comprehensive Husbandry Protocol for Corydoras Catfish and Many Other Amazonian Species.J Am Assoc Lab Anim Sci. 2024 Sep 2;63(5):472-9. doi: 10.30802/AALAS-JAALAS-24-039. Online ahead of print. J Am Assoc Lab Anim Sci. 2024. PMID: 39223031 Free PMC article.
-
Postembryonic staging of wild-type goldfish, with brief reference to skeletal systems.Dev Dyn. 2015 Dec;244(12):1485-518. doi: 10.1002/dvdy.24340. Epub 2015 Sep 18. Dev Dyn. 2015. PMID: 26316229 Free PMC article.
References
-
- Arocha-Piñago CL, Guerrero B. Lonomia genus caterpillar envenomation: Clinical and biological aspects. Haemostasis. 2001;31:288–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

