Inhibition of HIV-1 integrase nuclear import and replication by a peptide bearing integrase putative nuclear localization signal
- PMID: 19961612
- PMCID: PMC3224947
- DOI: 10.1186/1742-4690-6-112
Inhibition of HIV-1 integrase nuclear import and replication by a peptide bearing integrase putative nuclear localization signal
Abstract
Background: The integrase (IN) of human immunodeficiency virus type 1 (HIV-1) has been implicated in different steps during viral replication, including nuclear import of the viral pre-integration complex. The exact mechanisms underlying the nuclear import of IN and especially the question of whether it bears a functional nuclear localization signal (NLS) remain controversial.
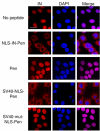
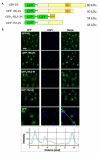
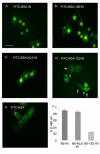
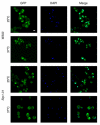
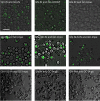
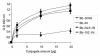
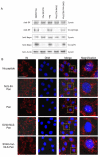
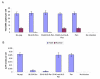
Results: Here, we studied the nuclear import pathway of IN by using multiple in vivo and in vitro systems. Nuclear import was not observed in an importin alpha temperature-sensitive yeast mutant, indicating an importin alpha-mediated process. Direct interaction between the full-length IN and importin alpha was demonstrated in vivo using bimolecular fluorescence complementation assay (BiFC). Nuclear import studies in yeast cells, with permeabilized mammalian cells, or microinjected cultured mammalian cells strongly suggest that the IN bears a NLS domain located between residues 161 and 173. A peptide bearing this sequence -NLS-IN peptide- inhibited nuclear accumulation of IN in transfected cell-cycle arrested cells. Integration of viral cDNA as well as HIV-1 replication in viral cell-cycle arrested infected cells were blocked by the NLS-IN peptide.
Conclusion: Our present findings support the view that nuclear import of IN occurs via the importin alpha pathway and is promoted by a specific NLS domain. This import could be blocked by NLS-IN peptide, resulting in inhibition of viral infection, confirming the view that nuclear import of the viral pre-integration complex is mediated by viral IN.
Figures
Similar articles
-
Transportin 3 and importin α are required for effective nuclear import of HIV-1 integrase in virus-infected cells.Nucleus. 2010 Sep-Oct;1(5):422-31. doi: 10.4161/nucl.1.5.12903. Nucleus. 2010. PMID: 21326825 Free PMC article.
-
A synthetic peptide bearing the HIV-1 integrase 161-173 amino acid residues mediates active nuclear import and binding to importin alpha: characterization of a functional nuclear localization signal.J Mol Biol. 2004 Mar 5;336(5):1117-28. doi: 10.1016/j.jmb.2003.11.057. J Mol Biol. 2004. PMID: 15037073
-
Identification of critical motifs within HIV-1 integrase required for importin α3 interaction and viral cDNA nuclear import.J Mol Biol. 2011 Jul 29;410(5):847-62. doi: 10.1016/j.jmb.2011.04.011. J Mol Biol. 2011. PMID: 21763491
-
HIV-1 nuclear import: in search of a leader.Front Biosci. 1997 Dec 1;2:d578-87. doi: 10.2741/a213. Front Biosci. 1997. PMID: 9366553 Review.
-
HIV type 1 integrase inhibitors: from basic research to clinical implications.AIDS Rev. 2008 Jul-Sep;10(3):172-89. AIDS Rev. 2008. PMID: 18820719 Review.
Cited by
-
Transportin 3 and importin α are required for effective nuclear import of HIV-1 integrase in virus-infected cells.Nucleus. 2010 Sep-Oct;1(5):422-31. doi: 10.4161/nucl.1.5.12903. Nucleus. 2010. PMID: 21326825 Free PMC article.
-
Molecular characterization of HIV-1 genome in fission yeast Schizosaccharomyces pombe.Cell Biosci. 2015 Aug 25;5:47. doi: 10.1186/s13578-015-0037-7. eCollection 2015. Cell Biosci. 2015. PMID: 26309721 Free PMC article.
-
Microbial Natural Product Alternariol 5-O-Methyl Ether Inhibits HIV-1 Integration by Blocking Nuclear Import of the Pre-Integration Complex.Viruses. 2017 May 10;9(5):105. doi: 10.3390/v9050105. Viruses. 2017. PMID: 28489061 Free PMC article.
-
Strategies to inhibit viral protein nuclear import: HIV-1 as a target.Biochim Biophys Acta. 2011 Sep;1813(9):1646-53. doi: 10.1016/j.bbamcr.2010.07.010. Epub 2010 Aug 16. Biochim Biophys Acta. 2011. PMID: 20719241 Free PMC article. Review.
-
Prospective strategies for targeting HIV-1 integrase function.Future Med Chem. 2010 Jul;2(7):1055-60. doi: 10.4155/fmc.10.205. Future Med Chem. 2010. PMID: 21359091 Free PMC article.
References
-
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. - PubMed
-
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. - PubMed
-
- Feldherr C, Akin D, Littlewood T, Stewart M. The molecular mechanism of translocation through the nuclear pore complex is highly conserved. J Cell Sci. 2002;115:2997–3005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

