MR imaging-guided interventions in the genitourinary tract: an evolving concept
- PMID: 19962090
- PMCID: PMC4072848
- DOI: 10.1016/j.mric.2009.09.002
MR imaging-guided interventions in the genitourinary tract: an evolving concept
Abstract
MR imaging-guided interventions are well established in routine patient care in many parts of the world. There are many approaches, depending on magnet design and clinical need, based on MR imaging providing excellent inherent tissue contrast without ionizing radiation risk for patients. MR imaging-guided minimally invasive therapeutic procedures have advantages over conventional surgical procedures. In the genitourinary tract, MR imaging guidance has a role in tumor detection, localization, and staging and can provide accurate image guidance for minimally invasive procedures. The advent of molecular and metabolic imaging and use of higher strength magnets likely will improve diagnostic accuracy and allow targeted therapy to maximize disease control and minimize side effects.
Figures
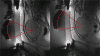





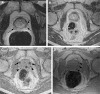




Republished from
-
MR imaging-guided interventions in the genitourinary tract: an evolving concept.Radiol Clin North Am. 2008 Jan;46(1):149-66, vii. doi: 10.1016/j.rcl.2008.01.004. Radiol Clin North Am. 2008. PMID: 18328885 Free PMC article. Review.
Similar articles
-
MR imaging-guided interventions in the genitourinary tract: an evolving concept.Radiol Clin North Am. 2008 Jan;46(1):149-66, vii. doi: 10.1016/j.rcl.2008.01.004. Radiol Clin North Am. 2008. PMID: 18328885 Free PMC article. Review.
-
Update to pulse sequences for interventional MR imaging.Magn Reson Imaging Clin N Am. 2005 Aug;13(3):415-29. doi: 10.1016/j.mric.2005.04.005. Magn Reson Imaging Clin N Am. 2005. PMID: 16084410
-
Pediatric MR-guided interventions.Eur J Radiol. 2005 Jan;53(1):57-66. doi: 10.1016/j.ejrad.2004.07.024. Eur J Radiol. 2005. PMID: 15607853 Review.
-
MR imaging-controlled focused ultrasound ablation: a noninvasive image-guided surgery.Magn Reson Imaging Clin N Am. 2005 Aug;13(3):545-60. doi: 10.1016/j.mric.2005.04.008. Magn Reson Imaging Clin N Am. 2005. PMID: 16084419 Review.
-
First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system.Med Phys. 2009 Jun;36(6):2084-8. doi: 10.1118/1.3125662. Med Phys. 2009. PMID: 19610297
Cited by
-
Non-linear adaptive controllers for an over-actuated pneumatic MR-compatible stepper.Med Biol Eng Comput. 2013 Jul;51(7):799-809. doi: 10.1007/s11517-013-1050-9. Epub 2013 Feb 22. Med Biol Eng Comput. 2013. PMID: 23430329
-
Focused ultrasound surgery in oncology: overview and principles.Radiology. 2011 Apr;259(1):39-56. doi: 10.1148/radiol.11100155. Radiology. 2011. PMID: 21436096 Free PMC article. Review.
-
Investigating the role of DCE-MRI, over T2 and DWI, in accurate PI-RADS v2 assessment of clinically significant peripheral zone prostate lesions as defined at radical prostatectomy.Abdom Radiol (NY). 2019 Apr;44(4):1520-1527. doi: 10.1007/s00261-018-1807-6. Abdom Radiol (NY). 2019. PMID: 30361870 Free PMC article.
References
-
- Stewart AJ, Viswanathan AN. Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer. 2006;107(5):908–15. - PubMed
-
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–8. - PubMed
-
- Ravina J, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671–2. - PubMed
-
- Fry WJ, Barnard JW, Fry FJ. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med. 1955;34:413–23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

