Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody
- PMID: 19962307
- PMCID: PMC3349232
- DOI: 10.1016/j.cub.2009.10.050
Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody
Abstract
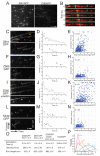
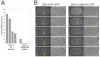
Cytokinesis in animal cells requires the central spindle and midbody, which contain prominent microtubule bundles. Centralspindlin, a heterotetrameric complex consisting of kinesin-6 and RhoGAP (Rho-family GTPase-activating protein) subunits, is essential for the formation of these structures. Centralspindlin becomes precisely localized to the central spindle, where it promotes the equatorial recruitment of important cytokinetic regulators. These include ECT2, the activator of the small GTPase RhoA, which controls cleavage furrow formation and ingression. Centralspindlin's own RhoGAP domain also contributes to furrow ingression. Finally, centralspindlin facilitates recruitment of the chromosome passenger complex and factors that control abscission. Despite the importance of localized accumulation of centralspindlin, the mechanism by which this motor protein complex suddenly concentrates to the center of interpolar microtubule bundles during anaphase is unclear. Here, we show that centralspindlin travels along central spindle microtubules as higher-order clusters. Clustering of centralspindlin is critical for microtubule bundling and motility along microtubules in vitro and for midbody formation in vivo. These data support a positive feedback loop of centralspindlin clustering and microtubule organization that may underlie its distinctive localization during cytokinesis.
Figures



Similar articles
-
Centralspindlin: at the heart of cytokinesis.Cytoskeleton (Hoboken). 2012 Nov;69(11):882-92. doi: 10.1002/cm.21065. Epub 2012 Sep 21. Cytoskeleton (Hoboken). 2012. PMID: 22927365 Free PMC article. Review.
-
Kinesin-like protein KIF18A is required for faithful coordination of chromosome congression with cytokinesis.FEBS J. 2025 Aug;292(15):3910-3925. doi: 10.1111/febs.70019. Epub 2025 Feb 15. FEBS J. 2025. PMID: 39954259
-
Centralspindlin in Rappaport's cleavage signaling.Semin Cell Dev Biol. 2016 May;53:45-56. doi: 10.1016/j.semcdb.2016.03.006. Epub 2016 Mar 7. Semin Cell Dev Biol. 2016. PMID: 26964770 Review.
-
Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis.Mol Biol Cell. 2006 Sep;17(9):3881-96. doi: 10.1091/mbc.e06-01-0015. Epub 2006 Jun 21. Mol Biol Cell. 2006. PMID: 16790497 Free PMC article.
-
Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis.Nature. 2012 Dec 13;492(7428):276-9. doi: 10.1038/nature11773. Nature. 2012. PMID: 23235882
Cited by
-
p53 and cell cycle dependent transcription of kinesin family member 23 (KIF23) is controlled via a CHR promoter element bound by DREAM and MMB complexes.PLoS One. 2013 May 1;8(5):e63187. doi: 10.1371/journal.pone.0063187. Print 2013. PLoS One. 2013. PMID: 23650552 Free PMC article.
-
Integrative analyses of gene expression profile reveal potential crucial roles of mitotic cell cycle and microtubule cytoskeleton in pulmonary artery hypertension.BMC Med Genomics. 2020 Jun 26;13(1):86. doi: 10.1186/s12920-020-00740-x. BMC Med Genomics. 2020. PMID: 32586319 Free PMC article.
-
Polar relaxation by dynein-mediated removal of cortical myosin II.J Cell Biol. 2020 Aug 3;219(8):e201903080. doi: 10.1083/jcb.201903080. J Cell Biol. 2020. PMID: 32497213 Free PMC article.
-
Rho-guanine nucleotide exchange factors during development: Force is nothing without control.Small GTPases. 2010 Jul;1(1):28-43. doi: 10.4161/sgtp.1.1.12672. Small GTPases. 2010. PMID: 21686118 Free PMC article.
-
Diversity of developing peripheral glia revealed by single-cell RNA sequencing.Dev Cell. 2021 Sep 13;56(17):2516-2535.e8. doi: 10.1016/j.devcel.2021.08.005. Epub 2021 Aug 31. Dev Cell. 2021. PMID: 34469751 Free PMC article.
References
-
- Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. - PubMed
-
- Somers WG, Saint R. A RhoGEF and Rho family GTPaseactivating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

