Developmentally regulated impediments to skin reinnervation by injured peripheral sensory axon terminals
- PMID: 19962310
- PMCID: PMC2805760
- DOI: 10.1016/j.cub.2009.10.051
Developmentally regulated impediments to skin reinnervation by injured peripheral sensory axon terminals
Abstract
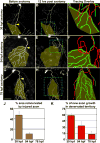
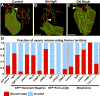
The structural plasticity of neurites in the central nervous system (CNS) diminishes dramatically after initial development, but the peripheral nervous system (PNS) retains substantial plasticity into adulthood. Nevertheless, functional reinnervation by injured peripheral sensory neurons is often incomplete [1-6]. To investigate the developmental control of skin reinnervation, we imaged the regeneration of trigeminal sensory axon terminals in live zebrafish larvae following laser axotomy. When axons were injured during early stages of outgrowth, regenerating and uninjured axons grew into denervated skin and competed with one another for territory. At later stages, after the establishment of peripheral arbor territories, the ability of uninjured neighbors to sprout diminished severely, and although injured axons reinitiated growth, they were repelled by denervated skin. Regenerating axons were repelled specifically by their former territories, suggesting that local inhibitory factors persist in these regions. Antagonizing the function of several members of the Nogo receptor (NgR)/RhoA pathway improved the capacity of injured axons to grow into denervated skin. Thus, as in the CNS, impediments to reinnervation in the PNS arise after initial establishment of axon arbor structure.
Figures


Similar articles
-
Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning.Development. 2010 Dec;137(23):3985-94. doi: 10.1242/dev.053611. Epub 2010 Nov 1. Development. 2010. PMID: 21041367 Free PMC article.
-
Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin.PLoS Biol. 2011 May;9(5):e1000621. doi: 10.1371/journal.pbio.1000621. Epub 2011 May 24. PLoS Biol. 2011. PMID: 21629674 Free PMC article.
-
Injury-induced remodelling and regeneration of the ribbon presynaptic terminal in vitro.J Neurocytol. 1996 Oct;25(10):597-613. doi: 10.1007/BF02284827. J Neurocytol. 1996. PMID: 8971639
-
Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system.Motor Control. 2009 Oct;13(4):412-41. doi: 10.1123/mcj.13.4.412. Motor Control. 2009. PMID: 20014648 Review.
-
Mechanisms and consequences of microglial responses to peripheral axotomy.Front Biosci (Schol Ed). 2011 Jun 1;3(3):857-68. doi: 10.2741/192. Front Biosci (Schol Ed). 2011. PMID: 21622237 Review.
Cited by
-
No simpler than mammals: axon and dendrite regeneration in Drosophila.Genes Dev. 2012 Jul 15;26(14):1509-14. doi: 10.1101/gad.198150.112. Genes Dev. 2012. PMID: 22802524 Free PMC article.
-
Dendrite injury triggers DLK-independent regeneration.Cell Rep. 2014 Jan 30;6(2):247-53. doi: 10.1016/j.celrep.2013.12.022. Epub 2014 Jan 9. Cell Rep. 2014. PMID: 24412365 Free PMC article.
-
In vivo nerve-macrophage interactions following peripheral nerve injury.J Neurosci. 2012 Mar 14;32(11):3898-909. doi: 10.1523/JNEUROSCI.5225-11.2012. J Neurosci. 2012. PMID: 22423110 Free PMC article.
-
A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration.Cell Death Differ. 2015 May;22(5):731-42. doi: 10.1038/cdd.2014.164. Epub 2014 Oct 17. Cell Death Differ. 2015. PMID: 25323584 Free PMC article.
-
Mapping the m1A, m5C, m6A and m7G methylation atlas in zebrafish brain under hypoxic conditions by MeRIP-seq.BMC Genomics. 2022 Feb 8;23(1):105. doi: 10.1186/s12864-022-08350-w. BMC Genomics. 2022. PMID: 35135476 Free PMC article.
References
-
- Chen ZL, Yu WM, Strickland S. Peripheral Regeneration. Annu Rev Neurosci 2007 - PubMed
-
- Hoke A. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–454. - PubMed
-
- Navarro X, Verdu E, Wendelschafer-Crabb G, Kennedy WR. Immunohistochemical study of skin reinnervation by regenerative axons. J Comp Neurol. 1997;380:164–174. - PubMed
-
- Rajan B, Polydefkis M, Hauer P, Griffin JW, McArthur JC. Epidermal reinnervation after intracutaneous axotomy in man. J Comp Neurol. 2003;457:24–36. - PubMed
-
- Speidel CC. In vivo studies of myelinated nerve fibers. Int Rev Cytol. 1964;16:173–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

