The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation
- PMID: 19962315
- PMCID: PMC2828323
- DOI: 10.1016/j.cub.2009.10.062
The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation
Abstract
Background: Actin polymerization by Arp2/3 complex must be tightly regulated to promote clathrin-mediated endocytosis. Although many Arp2/3 complex activators have been identified, mechanisms for its negative regulation have remained more elusive. To address this, we analyzed the yeast arp2-7 allele, which is biochemically unique in causing unregulated actin assembly in vitro in the absence of Arp2/3 activators.
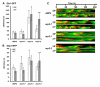
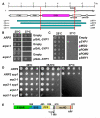
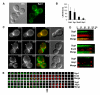
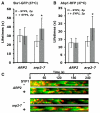
Results: We examined endocytosis in arp2-7 mutants by live-cell imaging of Sla1-GFP, a coat marker, and Abp1-RFP, which marks the later actin phase of endocytosis. Sla1-GFP and Abp1-RFP lifetimes were accelerated in arp2-7 mutants, which is opposite to actin nucleation-impaired arp2 alleles or deletions of Arp2/3 activators. We performed a screen for multicopy suppressors of arp2-7 and identified SYP1, an FCHO1 homolog, which contains F-BAR and AP-2micro homology domains. Overexpression of SYP1 in arp2-7 cells slowed Sla1-GFP lifetimes closer to wild-type cells. Further, purified Syp1 directly inhibited Las17/WASp stimulation of Arp2/3 complex-mediated actin assembly in vitro. This activity was mapped to a fragment of Syp1 located between its F-BAR and AP-2micro homology domains and depends on sequences in Las17/WASp outside of the VCA domain.
Conclusions: Together, these data identify Syp1 as a novel negative regulator of WASp-Arp2/3 complex that helps choreograph the precise timing of actin assembly during endocytosis.
Figures
Similar articles
-
SLAC, a complex between Sla1 and Las17, regulates actin polymerization during clathrin-mediated endocytosis.Mol Biol Cell. 2012 Nov;23(21):4256-72. doi: 10.1091/mbc.E11-12-1022. Epub 2012 Sep 12. Mol Biol Cell. 2012. PMID: 22973053 Free PMC article.
-
A second Las17 monomeric actin-binding motif functions in Arp2/3-dependent actin polymerization during endocytosis.Traffic. 2015 Apr;16(4):379-97. doi: 10.1111/tra.12259. Epub 2015 Feb 24. Traffic. 2015. PMID: 25615019 Free PMC article.
-
Lsb1 is a negative regulator of las17 dependent actin polymerization involved in endocytosis.PLoS One. 2013;8(4):e61147. doi: 10.1371/journal.pone.0061147. Epub 2013 Apr 8. PLoS One. 2013. PMID: 23577202 Free PMC article.
-
WASP family proteins, more than Arp2/3 activators.Biochem Soc Trans. 2016 Oct 15;44(5):1339-1345. doi: 10.1042/BST20160176. Biochem Soc Trans. 2016. PMID: 27911716 Free PMC article. Review.
-
New insights into the regulation and cellular functions of the ARP2/3 complex.Nat Rev Mol Cell Biol. 2013 Jan;14(1):7-12. doi: 10.1038/nrm3492. Epub 2012 Dec 5. Nat Rev Mol Cell Biol. 2013. PMID: 23212475 Review.
Cited by
-
Analysis of yeast endocytic site formation and maturation through a regulatory transition point.Mol Biol Cell. 2012 Feb;23(4):657-68. doi: 10.1091/mbc.E11-02-0108. Epub 2011 Dec 21. Mol Biol Cell. 2012. PMID: 22190733 Free PMC article.
-
Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily.Cell Mol Life Sci. 2011 Dec;68(24):3983-93. doi: 10.1007/s00018-011-0768-5. Epub 2011 Jul 17. Cell Mol Life Sci. 2011. PMID: 21769645 Free PMC article. Review.
-
Rho1- and Pkc1-dependent phosphorylation of the F-BAR protein Syp1 contributes to septin ring assembly.Mol Biol Cell. 2015 Sep 15;26(18):3245-62. doi: 10.1091/mbc.E15-06-0366. Epub 2015 Jul 15. Mol Biol Cell. 2015. PMID: 26179915 Free PMC article.
-
Identification of an ATP-controlled allosteric switch that controls actin filament nucleation by Arp2/3 complex.Nat Commun. 2016 Jul 15;7:12226. doi: 10.1038/ncomms12226. Nat Commun. 2016. PMID: 27417392 Free PMC article.
-
Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning.Nat Cell Biol. 2012 Apr 8;14(5):488-501. doi: 10.1038/ncb2473. Nat Cell Biol. 2012. PMID: 22484487 Free PMC article.
References
-
- Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. - PubMed
-
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. - PubMed
-
- Girao H, Geli MI, Idrissi FZ. Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 2008;582:2112–2119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32-GM084677/GM/NIGMS NIH HHS/United States
- T32 GM007231/GM/NIGMS NIH HHS/United States
- R01-GM083137/GM/NIGMS NIH HHS/United States
- T32 GM007596/GM/NIGMS NIH HHS/United States
- F32 GM084677/GM/NIGMS NIH HHS/United States
- T32-GM07231/GM/NIGMS NIH HHS/United States
- R01 GM060979/GM/NIGMS NIH HHS/United States
- R01-GM055796/GM/NIGMS NIH HHS/United States
- R01 GM063691/GM/NIGMS NIH HHS/United States
- R01-GM063691/GM/NIGMS NIH HHS/United States
- R01 GM083137/GM/NIGMS NIH HHS/United States
- R01-GM060979/GM/NIGMS NIH HHS/United States
- R01 GM055796/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

